Abstract
The purpose of this study was to investigate the formulation variables influencing the drug release from the layered tablets containing chitosan and xanthan gum as matrix component. Increasing the amount of lactose could diminish pH sensitive release behavior of these matrix tablets. Effect of formulation variables on drug release from the prepared three-layered matrix tablets was investigated. The amount of drug loading did not affect the drug release which was influenced by the hydrodynamic force and the matrix composition. An increase in stirring rate correspondingly increased the release rate. Moreover, incorporation of soluble diluents in core or barrier could enhance the drug release. Least square fitting the experimental dissolution data to the mathematical expressions (power law, first order, Higuchi’s and zero order) was carried out to study the drug release mechanism. Most dissolution profiles of the prepared three-layered tablets provided a better fit to zero order kinetic than to first order kinetic and Higuchi’s equation.
Key words: chitosan, drug release, formulation variables, layered matrix, xanthan gum
INTRODUCTION
Chitosan–xanthan gum as matrix materials in three-layered tablet was claimed to sustain the release of water soluble drug such as propranolol HCl and shift the release pattern approach to zero order (1). Chlorpheniramine maleate sustained release tablet prepared by hot-melt extrusion containing these two matrix materials was also reported (2). The drug release exhibited pH and buffer species independent attributable to slow media uptake into tablet that resulted from the molten state of preparation process and the inter- and intra-molecular hydrogelation of the matrix materials.
Different core or barrier composition of three-layered matrix tablets has been investigated. Linear release profiles could be obtained by applying hydrophilic barrier layers on both faces of a hydrophobic matrix tablet, or by applying a hydrophilic barrier layer on one face and a hydrophobic barrier layer on the other face of a matrix tablet (3). Hydroxypropyl methylcellulose (HPMC) as barrier was more efficient in reducing drug delivery than poly(ethylene oxide) (PEO) due to the viscosity generated from polymer (4). Swellable barrier exhibited stronger release modulation efficiency and was more suitable to modify the release of highly water soluble drugs, whilst erodible barrier exhibited a time-dependent coating effect that provided better control release of sparingly soluble drugs (5).
Several formulation and processing variables, such as the presence of diluents and compression force, could modify the drug release from the matrices (6,7) whereas loading dose of diltiazem HCl in three-layered tablets prepared using PEO and HPMC could not (8). For environmental variables, an increase in stirring rate during dissolution study enhanced the drug release, but the linearity of release profiles remained unaltered (8). Moreover, agitation intensity, hydrodynamic force and hydrodynamic condition were reported to influence the drug dissolution rate (9).
Although considerable research has been done on aforementioned manner, the effect of formulation variables on the drug release from layered matrix as controlled release device containing two polymers which could form polyelectrolyte complex, especially chitosan and xanthan gum, has not yet been addressed. The study of formulation variables influencing drug release from this controlled release device has been an important aspect in the development stage. Therefore, the effects of compression force, hydrodynamic force, drug loading and type and amount of diluents on physical properties and release of propranolol HCl from three-layered tablet comprising chitosan and xanthan gum were investigated in this study.
MATERIALS AND METHODS
Materials
Xanthan gum (Xantural 75, CP Kelco U.S., Inc. Chicago, USA.), propranolol hydrochloride (Batch No. 941002, China National Chemical Imp. & Exp., Shanghai, China), lactose (Wyndale, Hawera, New Zealand), ethylcellulose (EC) (Ethocel Standard 45 Premium, Lot MC 23013t01, Dow Chemical Company, Michigan, USA.), Avicel PH 101 (Lot no. 2784, Asahi Chemical Industry, Japan), polyethylene glycol 4000 (PC Drug, Thailand), malic acid and sodium bicarbonate (Batch Nos. AF409282 and AF310196, respectively, Asia Pacific Specialty Chemicals Ltd., NSW, Australia) and sodium chloride (Lot k20420804, Merk, E Merck, Darmstadt, Germany) were used as received. Chitosan (Aqua Premier, Chonburi, Thailand) with 99.3% degree of deacetylation and molecular weight 70 kDa, magnesium stearate (Lot No. MAF 07, P. C. Drug Center Co.) and phytowax®Olive 14L 48, hydrogenated myristyl ester of olive oil, (Sophim, Parc de la Cassine, France) were passed through sieve No. 60 before used. All other reagents were of AR grade.
Preparation of Matrix Tablets
The 400-mg tablets containing propranolol HCl were prepared by direct compression method. The single layered tablets containing 1:1 chitosan and xanthan gum with various amount of lactose were prepared according to the composition in Table I. The amount of lactose was varied from 25% to 100% of the matrix component excluding the amount of drug and magnesium stearate. The concentration of drug was kept constant at 20% by weight (80-mg/tablet). All ingredients were mixed for 5 min using mortar and pestle. Then the drug blended powder was compressed into a tablet at a compression force of two tons using 13 mm round, flat and plain punches on a hydraulic press (Carver Press, WI).
Table I.
Composition of Tablets Containing Different Amount of Lactose
| Substances | Formulation (%,w/w) | ||||
|---|---|---|---|---|---|
| A0 | A25 | A50 | A75 | A100 | |
| Propranolol HCl | 20 | 20 | 20 | 20 | 20 |
| Chitosan | 39 | 29.25 | 19.5 | 9.75 | – |
| Xanthan gum | 39 | 29.25 | 19.5 | 9.75 | – |
| Lactose | – | 19.50 | 39 | 58.5 | 78 |
| Magnesium stearate | 2 | 2 | 2 | 2 | 2 |
The layered matrix tablets were prepared by adding 100-mg of the powder mixture without drug in the die cavity and slightly compressed for uniform spreading. The 200-mg of the powder mixture with drug was placed over the first layer and again slightly compressed for uniform spreading. Another 100-mg of the powder mixture without drug was subsequently placed and compressed with compressional force using a hydraulic press to obtain the three-layered tablet. The dwell time after achieving target pressure achieved was 10 s. The compression force was also varied as 1, 2, 3 and 4 tons. To study the effect of formulation variables, different amount and type of diluents were investigated. This included the tablets containing the middle layer of 200-mg of 1:1 chitosan and xanthan gum and 75% various diluents (as shown in Table II) with the 100-mg upper and 100-mg lower barriers of the same formula of core without drug. Additionally, the tablets were also studied that contained the middle layer of 200-mg of 1:1 chitosan:xanthan gum and 55% lactose and 20% other substances (Table III) with the 100-mg upper and 100-mg lower barriers containing 1:1 chitosan:xanthan gum and 75% lactose. Moreover, the effect of drug loading dose (40, 80, 100 and 120 mg) was also investigated.
Table II.
Composition of Three-Layered Tablets Containing 200-mg in Middle Layer of 1:1 Chitosan and Xanthan Gum and 75% other Diluents and (B) 55% Lactose and 20% Other Diluents
| Substances | Formulation (%,w/w) | ||||
|---|---|---|---|---|---|
| A75 | B75 | C75 | D75 | E75 | |
| Propranolol HCl | 20 | 20 | 20 | 20 | 20 |
| Chitosan | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 |
| Xanthan gum | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 |
| Lactose | 58.5 | ||||
| Microcrystalline cellulose | 58.5 | ||||
| PEG 4000 | 58.5 | ||||
| Phytowax | 58.5 | ||||
| Ethyl cellulose | 58.5 | ||||
| Magnesium stearate | 2 | 2 | 2 | 2 | 2 |
With the 100-mg upper and 100-mg lower barriers of the same formula without drug
Table III.
Composition of Three-Layered Tablets Containing 200-mg in Middle Layer of 1:1 Chitosan and Xanthan Gum and 55% Lactose and 20% Other Diluents
| Substances | Formulation (%,w/w) | |||||
|---|---|---|---|---|---|---|
| A55B20 | A55C20 | A55D20 | A55F20 | A55G20 | A55H20 | |
| Propranolol HCl | 20 | 20 | 20 | 20 | 20 | 20 |
| Chitosan | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 |
| Xanthan gum | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 | 9.75 |
| Lactose | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 |
| Microcrystalline cellulose | 15.6 | |||||
| PEG 4000 | 15.6 | |||||
| Phytowax | 15.6 | |||||
| Malic acid | 15.6 | |||||
| Sodium chloride | 15.6 | |||||
| Sodium bicarbonate | 15.6 | |||||
| Magnesium stearate | 2 | 2 | 2 | 2 | 2 | 2 |
With the 100-mg upper and 100-mg lower barriers containing 1:1 chitosan and xanthan gum, and 75% lactose
The Evaluation of Matrix Tablets
The hardness of tablets was determined using a hardness tester (Pharmatest, USA). The % friability was determined as the percent weight loss of 20 tablets after rotating for 100 revolutions in 4 min in a friabilitor (Yieheng Engineering, Bangkok, Thailand). Drug release was undertaken using a dissolution apparatus (Prolabo, France) with the basket method at 100 rpm and 900-ml HCl buffer pH 1.2 equilibrated at 37°C as dissolution fluid. Samples were collected at specific time intervals and assayed by a UV–Vis spectrophotometer (Perkin-Elmer, Germany) at a wavelength of 320 nm. To study the effect of the dissolution medium, drug release in pH change was also undertaken. The drug release in HCl buffer pH 1.2 was conducted for one and a half hour. Then the pH of medium was raised to 6.8 by adding 4.6 g sodium hydroxide, 3.06 g monobasic potassium phosphate and 4.005 g dibasic sodium phosphate until completing the operation at 12 h. During the drug release studies, the tablets were observed for physical integrity. To study the effect of hydrodynamic force on drug release, basket rotational speed of 25, 50, 100 and 150 rpm were operated.
Dissolution Profile Fitting
Least square fitting the experimental dissolution data (cumulative drug release >10% and up to 80%) to the mathematical equations (power law, first order, Higuchi’s and zero order) was carried out using a nonlinear computer programme, Scientist for Windows, version 2.1 (MicroMath Scientific Software, Salt Lake City, UT, USA). The coefficient of determination (r2) was used to indicate the degree of curve fitting. Goodness-of-fit was also evaluated using the Model Selection Criterion (MSC) (10), given below.
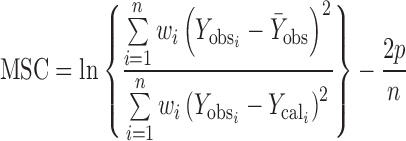 |
Where 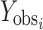 and
and 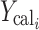 are observed and calculated values of the i-th point, respectively, and wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.
are observed and calculated values of the i-th point, respectively, and wi is the weight that applies to the i-th point, n is number of points and p is number of parameters.
RESULTS AND DISCUSSION
The Physical Properties of Matrix Tablets
The data on hardness and friability of the single- and the three-layered tablets are presented in Table IV. The hardness of tablet decreased as the amount of lactose was increased. The hardness of three-layered tablets tended to increase and the friability tended to diminish as the compression force was increased. Friability values of all formulations were less than 1%, except the formulation without chitosan and xanthan gum (A100), due to the low compressibility of lactose. Among three-layered tablets containing single diluent and those containing lactose with other diluent, ethyl cellulose (3L E75) and PEG 4000 (3L A55C20) provided the highest hardness, respectively. The latter might be due to binding property of PEG 4000 which could partially melt during compression. PEG 4000 as melting binder in carbamazepine fast-release tablets has been reported (11,12).
Table IV.
Physical Properties of Prepared Tablets
| Formulation | Thickness ± SD (mm) | Friability (%) | Hardness ± SD (N) |
|---|---|---|---|
| A0 | 2.12 ± 0.66 | 0.60 | 59.81 ± 3.84 |
| A25 | 2.45 ± 0.11 | 0.72 | 51.47 ± 4.64 |
| A50 | 2.36 ± 0.07 | 0.67 | 40.01 ± 3.92 |
| A75 | 2.37 ± 0.14 | 0.82 | 36.48 ± 4.93 |
| A100 | 2.47 ± 0.14 | 1.00 | 17.22 ± 5.16 |
| 3L A75 1 ton | 3.06 ± 0.29 | 0.62 | 9.63 ± 1.76 |
| 3L A75 2 ton | 2.42 ± 1.69 | 0.67 | 20.63 ± 2.86 |
| 3L A75 3 ton | 2.60 ± 0.05 | 0.40 | 24.43 ± 1.69 |
| 3L A75 4 ton | 2.52 ± 0.07 | 0.32 | 40.08 ± 4.60 |
| 3L 40-mg | 2.33 ± 0.02 | 0.64 | 10.20 ± 1.02 |
| 3L 80-mg | 2.42 ± 1.69 | 0.67 | 20.63 ± 2.86 |
| 3L100-mg | 2.20 ± 0.09 | 0.56 | 15.22 ± 2.53 |
| 3L 120-mg | 2.28 ± 0.04 | 0.64 | 16.44 ± 2.14 |
| 3L B75 | 2.74 ± 0.05 | 0.88 | 46.63 ± 7.28 |
| 3L C75 | 2.56 ± 0.07 | 0.98 | 48.63 ± 8.37 |
| 3L D75 | 2.93 ± 0.01 | 0.99 | 31.81 ± 3.26 |
| 3L E75 | 3.00 ± 0.09 | 0.69 | 55.02 ± 4.16 |
| 3L A55B20 | 2.74 ± 0.05 | 0.88 | 46.63 ± 7.28 |
| 3L A55C20 | 2.84 ± 0.02 | 0.98 | 52.81 ± 0.89 |
| 3L A55D20 | 2.93 ± 0.01 | 0.99 | 31.81 ± 3.28 |
| 3L A55F20 | 2.50 ± 0.06 | 0.89 | 19.63 ± 2.44 |
| 3L A55G20 | 2.54 ± 0.07 | 0.73 | 21.62 ± 2.04 |
| 3L A55H20 | 2.52 ± 0.06 | 0.83 | 24.62 ± 3.02 |
3L represents the three-layered tablet
In Vitro Drug Release Studies
Drug Release from Single Layer Tablet
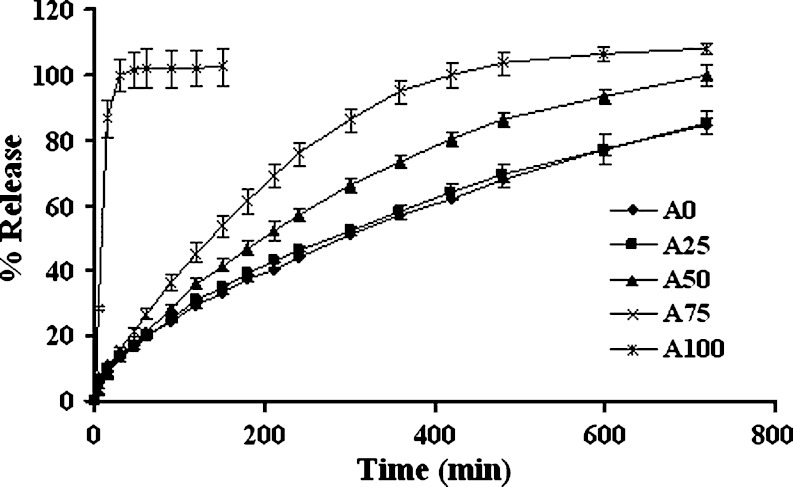
Increasing the amount of lactose in single layer tablet containing 1:1 chitosan:xanthan gum led to faster drug release as presented in Fig. 1. The effect of diluent on drug release from matrix devices was claimed to be mainly due to a change in hydrophilic gel expansion (13). This finding was in good agreement with the result of diphenhydramine HCl released from polyvinylacetate/polyvinylpyrrolidone matrix containing lactose or maize starch (14). Lactose, by its water soluble and hydrophilic nature, facilitated gel formation and the time taken for the dissolution medium to permeate to the core was shorter as the amount of this soluble substance was increased. Moreover, soluble substance acted as a channeling agent, by rapidly dissolving and easily diffusing outward, therefore allowing a decrease in tortuosity and/or an increase in the matrix porosity (15–17). These prepared tablets apparently released the drug slower than the tablet without chitosan and xanthan gum (A100). Ionic interaction between these two polymers as described by Dumitriu et al. (18,19) resulted in a decrease in the rate of polymer dissolution and the rate of solvent penetration. Therefore the drug diffusion into dissolution medium was diminished.
Fig. 1.
Dissolution profiles of single-layered tablets containing 1:1 chitosan:xanthan gum with different amount of lactose in HCl buffer pH 1.2. Each point represents the mean ± SD, n = 3
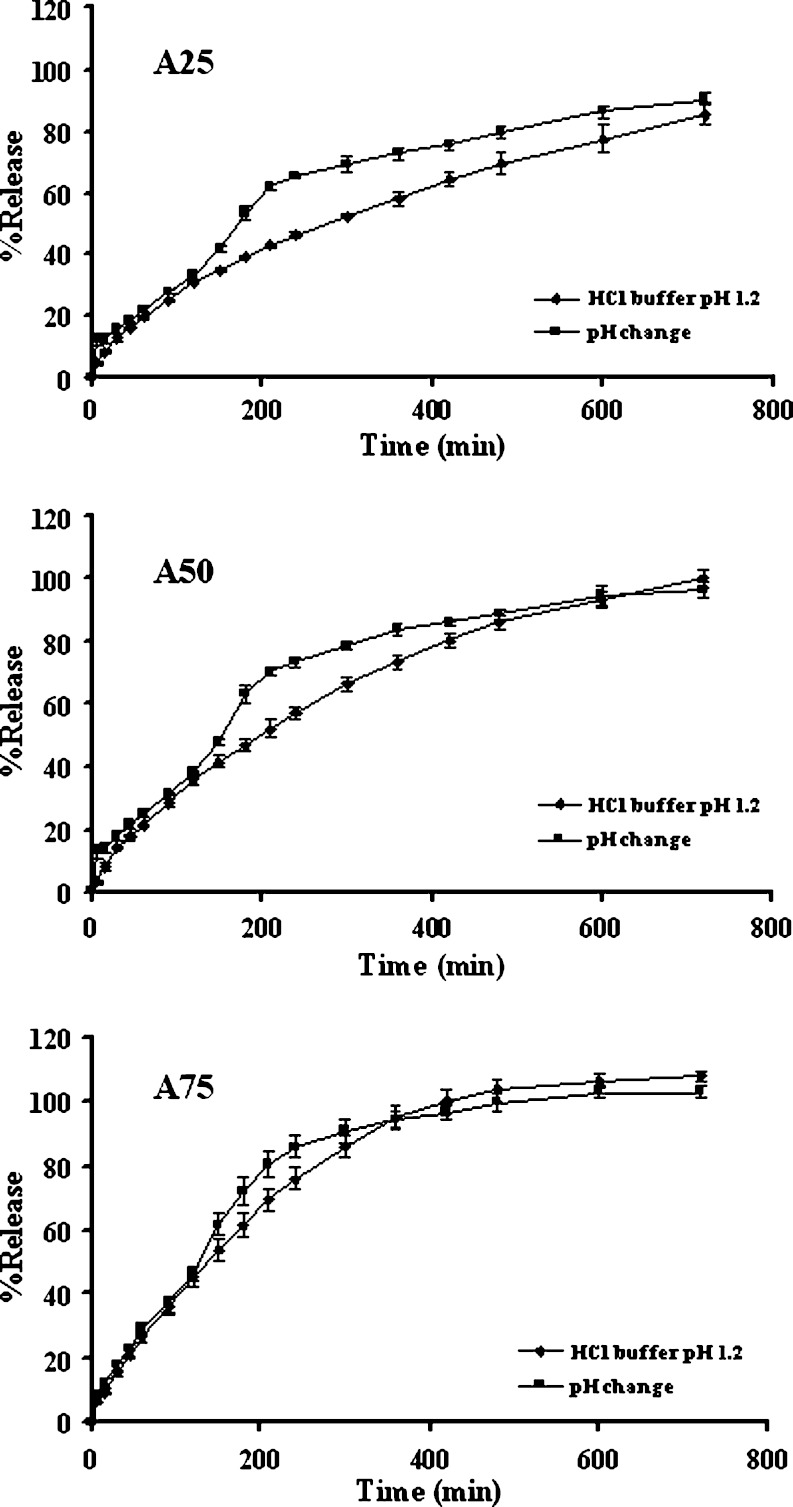
Curve upward of dissolution profile was observed during pH change as shown in Fig. 2. The pH dependence of drug release was decreased when the dissolution profile in acidic medium was closer to that in pH change media with the increased amount of lactose, especially, the tablet containing 75% lactose (A75). Therefore, lactose could adjust a pH sensitive property of polymeric matrix containing chitosan and xanthan gum due to the reduced amount of these polymers which played an important role in liquid uptake, erosion and swelling of the compact matrix. A common method to obtain the pH independent drug release is to adjust the environmental pH in dosage forms. Dissolution profile of drug from alginate matrices was different upon increasing the pH of the medium. In order to obtain pH-independent drug release, organic acid as pH-modifying agent was used to lower the pH in the intestine and thus resulted in high solubility of weakly basic drugs (14,18).
Fig. 2.
Dissolution profiles of single-layered tablets containing 1:1 chitosan:xanthan gum and lactose of 25% (A25), 50% (A50) and 75% (A75) in different dissolution fluids. Each point represents the mean ± SD, n = 3
Drug Release from Multiple Layers Tablet
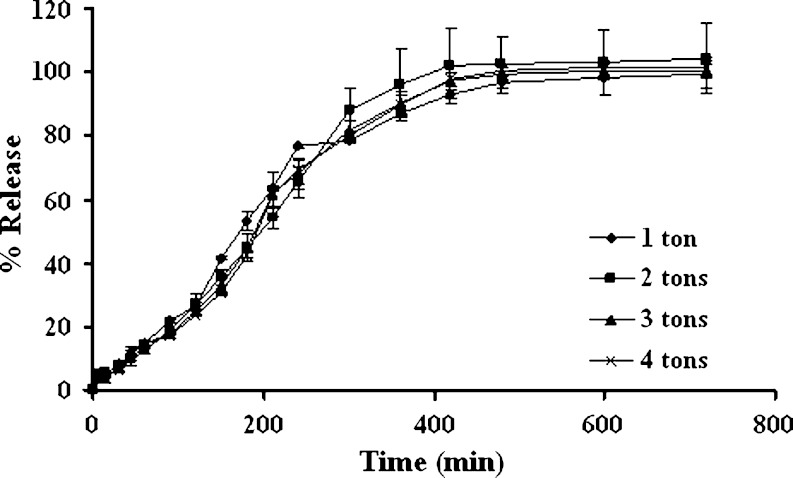
Compression force had no effect on the drug release from three-layered tablets as shown in Fig. 3 since the polymeric matrix could hydrate and form gel rapidly. Porosity of the hydrated matrix was independent of the initial porosity, thus the compression force posed to have little influence on drug release (20,21). Release of acetaminophen from HPMC/HPC matrix (22) and substituted amylose matrix (23) was not considerably influenced by changes in compression force. Conte et al. (24) also previously mentioned that compression force did not influence the system release performances even at very low force levels.
Fig. 3.
Effect of compression force on dissolution profiles of three-layered tablets containing 200 mg middle layer of 1:1 chitosan:xanthan gum and 75% lactose with the 100-mg upper and lower barriers in pH change media. Each point represents the mean ± SD, n = 3
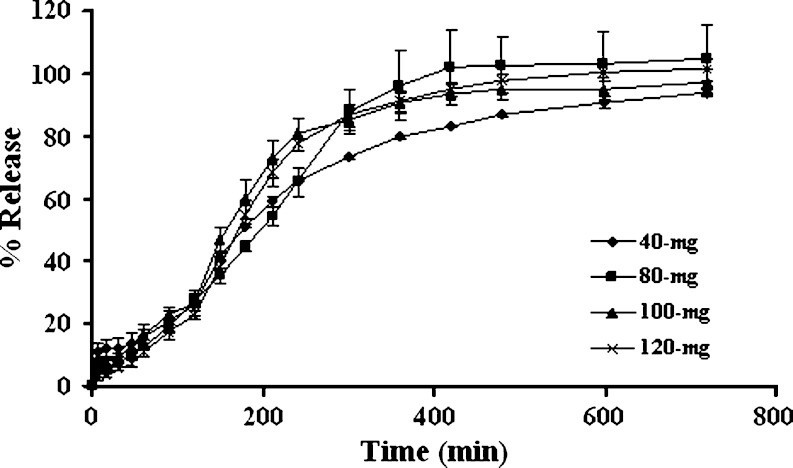
Varying the loading dose from 40 to 120-mg did not significantly change the drug release (Fig. 4), indicating that the penetration of medium into the matrix was not greatly altered. Moreover, the uptake of medium was dependent on the capacity of polymer to hydrate and the unique characteristic of system design rather than the incorporated drug. Similar result was also reported for the release of diltiazem HCl from three-layered tablet (8). Free water played an important role in the drug dissolution and mass transfer with the gel microstructure. As the drug loading was well above the drug solubility within the swollen/gelled matrix, variation in drug loading was not likely to produce the significant alteration of drug release rate (8).
Fig. 4.
Effect of drug loading on dissolution profiles of three-layered tablets containing 200 mg middle layer of 1:1 chitosan:xanthan gum and 75% lactose with the 100-mg upper and lower barriers in pH change media. Each point represents the mean ± SD, n = 3
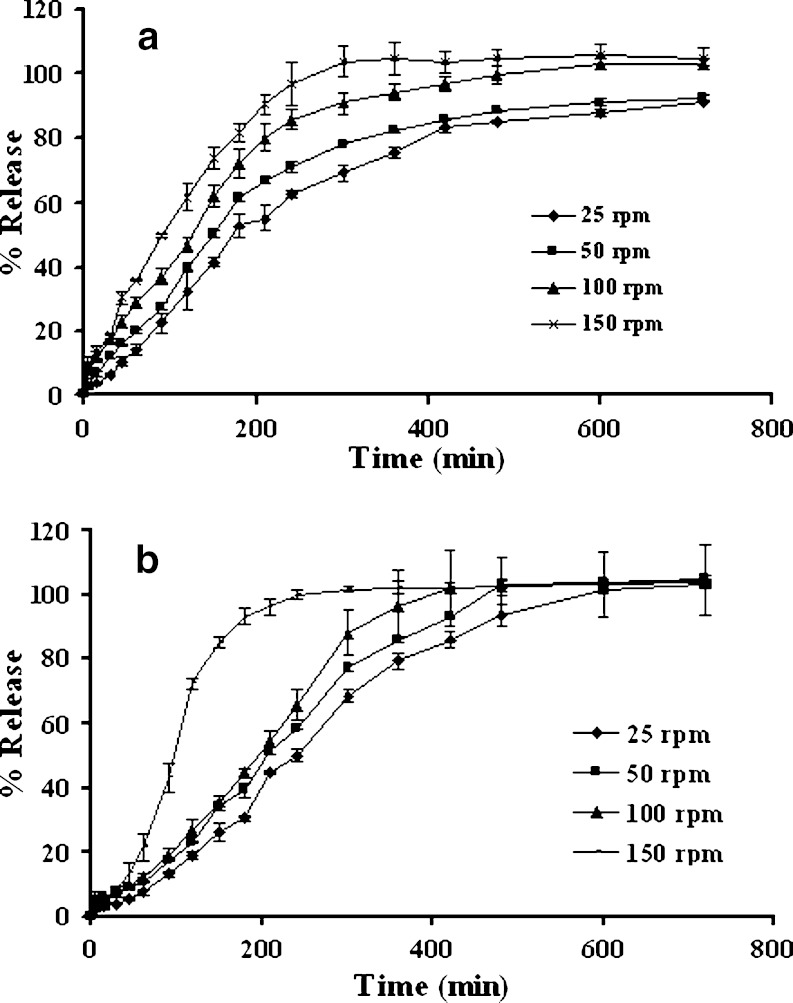
Increasing the basket rotational speed increased the drug dissolution from single and three-layered tablets as presented in Fig. 5a and b, respectively. This study demonstrated that hydrodynamic stress had an effect on dissolution rate, mass transfer rate, and film thickness underlying the dissolution process. As the stirring speed was excessive, the stagnant boundary layer effect was no longer the rate-limiting step in drug transport (8,25). In addition, an increase of mass transfer rate and a decrease of film thickness as the increment of basket rotation speed have been reported (9,26,27). Hydrodynamic stress and intensity caused greater attrition at the swollen periphery and was responsible for the increase in drug release rate.
Fig. 5.
Effect of rotational speed on dissolution profiles of single-layered tablets a and three-layered tablets b containing 1:1 chitosan:xanthan gum and 75% lactose in pH change media. Each point represents the mean ± SD, n = 3
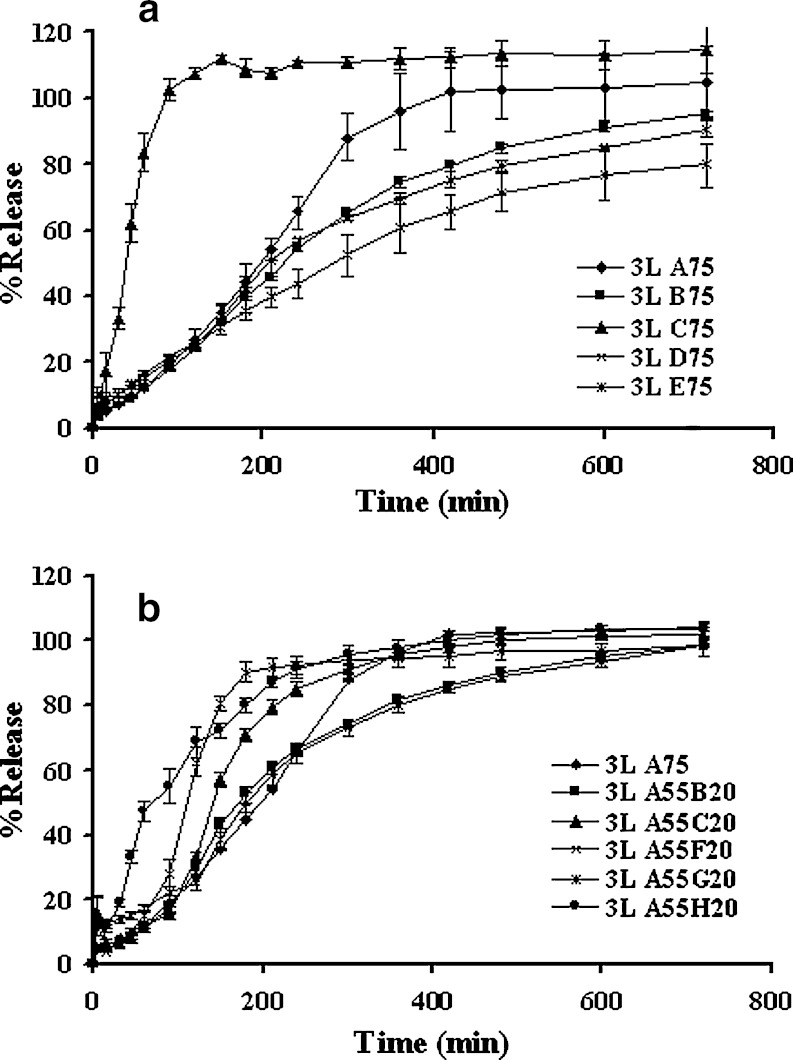
The drug dissolution profiles of three-layered tablets containing 1:1 chitosan:xanthan gum and 75% single additive are shown in Fig. 6a. The barrier layer contained the same formula as the core but without drug. Incorporation of PEG 4000 (3L C75) apparently showed the highest drug release owing to its high aqueous solubility. This was followed by the incorporation of lactose, Avicel, ethylcellulose and phytowax, respectively. Similar result of PEG was reported on theophylline tablet (28). It was evident from visual observation that Avicel formed a non-disintegrating matrix. Since the swelling of Avicel particles was limited to the amorphous domains and remained within the hydration matrix, the decrease of water sorption and erosion of this matrix was reported (29). Replacement of lactose by phytowax or ethyl cellulose could prolong the drug release. Owing to their insolubility, these diluents more obviously retarded the drug release than did lactose and microcrystalline cellulose. Similar result had been shown on the release of captopril from the ethylcellulose matrix tablets (30). The swellable barriers could form gels slowly and provide a strong protective effect for controlling the hydration process of the inner component of tablets. Thus, the drug released from middle layer could be modulated by the delayed diffusion from the two lateral layers as a result of polymer hydration and erosion. Generated gel barrier also effectively retarded the drug diffusion, therefore, this type of barrier was suitable to prolong the release of hydrophilic soluble drug. However, the barrier could be eroded in a well-defined period of dissolution time and its retardation efficiency was finished, whilst the swellable barrier could hydrate and gradually transform into gel that could still effectively control the drug release from the developed matrices as desired period of time.
Fig. 6.
Dissolution profiles of three-layered tablets containing 200-mg in middle layer of 1:1 chitosan and xanthan gum and a 75% other diluents and b 55% lactose and 20% other diluents in pH change media. Each point represents the mean ± SD, n = 3
Figure 6b exhibits the drug release profiles of three-layered tablets with 1:1 chitosan:xanthan gum and addition of 20% different additives with 55% lactose in the core component and 1:1 chitosan:xanthan gum and 75% lactose as barrier. It was found that the substitution of sodium bicarbonate (3L A55H20), malic acid (3L A55F20) and PEG 400 (3L A55C20) to lactose increased the drug release. Some electrolytes, fatty acids and bile salts could change the gel strength and interact with drug molecules; therefore the drug release could be modulated (31,32). The drug release from tablet containing Avicel PH101 (3L A55B20) and sodium chloride (3L A55G20) was similar to that from tablet containing only lactose (3L A75), but the latter showed greater drug release at the final stage. Highly hydrophilic compounds and ionized water soluble materials have been reported to interact with polymer by causing a partial dehydration of the polymeric molecules and thus the initial rapid diffusion of drug was limited (29,33). Although incorporation of sodium chloride could lower the gel point, increase the gel strength and decrease the hydration of polymer (34,35), these could be compensated and increase the drug release by its high solubility in aqueous system. Therefore, the hydrophilic characteristic of barrier layer played as important role in controlling the drug release from three-layered tablet which could be modulated by changing the composition of the barrier or the core layer. Excipients could have a dramatic effect on in vitro drug release when the systems were formulated with swellable polymers (36).
Dissolution Profile Fitting
The coefficient of determination (r2) and model selection criteria (MSC) from curve fitting to power law, first order, Higuchi’s and zero order equations are shown in Table V and the estimate parameters from curve fitting to power law equation are presented in Table VI. It was shown that fitting the experimental drug dissolution profiles to power law equation provided high r2 and MSC, indicating a superiority of this model.
Table V.
Comparison of Degree of Goodness-of-Fit from Curve Fitting of Drug Dissolution in pH Change Media to Different Release Models
| Tablet | Power law | First order | Higuchi’s | Zero order | ||||
|---|---|---|---|---|---|---|---|---|
| r 2 | MSC | r 2 | MSC | r 2 | MSC | r 2 | MSC | |
| A0a | 0.9998 | 8.05 | 0.9982 | 6.03 | 0.9884 | 4.19 | 0.9822 | 3.76 |
| A25a | 0.9994 | 7.05 | 0.9990 | 6.62 | 0.9963 | 5.32 | 0.9709 | 3.25 |
| A50a | 0.9996 | 7.28 | 0.9983 | 5.99 | 0.9876 | 4.03 | 0.9853 | 3.85 |
| A75a | 0.9999 | 8.87 | 0.9932 | 4.54 | 0.9826 | 3.61 | 0.9953 | 4.92 |
| A25 | 0.9613 | 2.85 | 0.9785 | 3.57 | 0.9456 | 2.65 | 0.9185 | 2.24 |
| A50 | 0.9832 | 3.59 | 0.9705 | 3.19 | 0.9270 | 2.28 | 0.9805 | 3.60 |
| A75 | 0.9961 | 4.79 | 0.9680 | 2.94 | 0.9235 | 2.07 | 0.9947 | 4.74 |
| 3L A75 1 ton | 0.9656 | 2.70 | 0.9461 | 2.45 | 0.8993 | 1.85 | 0.9641 | 2.88 |
| 3L A75 2 ton | 0.9999 | 8.87 | 0.9204 | 2.09 | 0.8490 | 1.45 | 0.9893 | 4.09 |
| 3L A75 3 ton | 0.9788 | 3.19 | 0.9163 | 2.04 | 0.7915 | 1.12 | 0.9734 | 3.18 |
| 3L A75 4 ton | 0.9719 | 2.91 | 0.9086 | 1.95 | 0.7346 | 0.88 | 0.9659 | 2.94 |
| 3L A75 40-mg | 0.9666 | 2.94 | 0.9438 | 2.57 | 0.9115 | 2.06 | 0.9664 | 3.09 |
| 3L A75 80-mg | 0.9999 | 8.87 | 0.9204 | 2.09 | 0.8490 | 1.45 | 0.9893 | 4.09 |
| 3L A75 100-mg | 0.9837 | 3.37 | 0.9149 | 1.96 | 0.8550 | 1.43 | 0.9744 | 3.17 |
| 3L A75 120-mg | 0.9873 | 3.51 | 0.9172 | 1.92 | 0.8391 | 1.26 | 0.9774 | 3.22 |
| A75 25 rpm | 0.9850 | 3.60 | 0.9934 | 4.63 | 0.9745 | 3.27 | 0.9501 | 2.60 |
| A75 50 rpm | 0.9828 | 3.46 | 0.9859 | 3.86 | 0.9572 | 2.75 | 0.9678 | 3.04 |
| A75 100 rpm | 0.9961 | 4.79 | 0.9680 | 2.94 | 0.9235 | 2.07 | 0.9947 | 4.74 |
| A75 150 rpm | 0.9967 | 4.87 | 0.9883 | 3.87 | 0.9396 | 2.23 | 0.9937 | 4.49 |
| 3L A75 25 rpm | 0.9908 | 3.94 | 0.9539 | 2.58 | 0.9046 | 1.85 | 0.9907 | 4.17 |
| 3L A75 50 rpm | 0.9966 | 5.03 | 0.9456 | 2.47 | 0.6965 | 0.75 | 0.9914 | 4.31 |
| 3L A75 100 rpm | 0.9999 | 8.87 | 0.9204 | 2.09 | 0.8490 | 1.45 | 0.9893 | 4.09 |
| 3L A75 150 rpm | 0.9874 | 3.38 | 0.9358 | 2.08 | 0.9537 | 1.26 | 0.9861 | 3.61 |
| 3L B75 | 0.9915 | 4.22 | 0.9845 | 3.80 | 0.9407 | 2.46 | 0.9836 | 3.75 |
| 3L C75 | 0.9956 | 4.22 | 0.9320 | 1.89 | 0.8598 | 1.16 | 0.9905 | 3.86 |
| 3L D75 | 0.9792 | 3.44 | 0.9832 | 3.80 | 0.9328 | 2.41 | 0.9639 | 3.04 |
| 3L E75 | 0.9859 | 3.86 | 0.9959 | 5.24 | 0.9799 | 3.64 | 0.9417 | 2.58 |
| 3L A55B20 | 0.9915 | 4.22 | 0.9845 | 3.80 | 0.9410 | 2.47 | 0.9836 | 3.75 |
| 3L A55C20 | 0.9956 | 4.22 | 0.9320 | 1.89 | 0.8644 | 1.20 | 0.9905 | 3.86 |
| 3L A55D20 | 0.9792 | 3.44 | 0.9832 | 3.80 | 0.9218 | 2.26 | 0.9639 | 3.04 |
| 3L A55F20 | 0.9793 | 2.68 | 0.9034 | 1.51 | 0.8354 | 1.00 | 0.9668 | 2.61 |
| 3L A55G20 | 0.9706 | 3.03 | 0.9476 | 2.61 | 0.8881 | 1.86 | 0.9703 | 3.18 |
| 3L A55H20 | 0.9798 | 3.15 | 0.9866 | 3.81 | 0.9428 | 2.36 | 0.9289 | 2.14 |
3L represents the three-layered tablet
aThe dissolution test performed in HCl buffer pH 1.2
Table VI.
Comparison of Estimate Parameters from Curve Fitting of Drug Dissolution in pH Change Media to Power Law Expression
| Tablet | k ± SD × 10-3 | n ± SD |
|---|---|---|
| A0a | 13.1166 ± 0.5855 | 0.6352 ± 0.0070 |
| A25a | 21.0820 ± 1.2986 | 0.5658 ± 0.0098 |
| A50a | 19.0240 ± 1.2682 | 0.5833 ± 0.0110 |
| A75a | 13.4090 ± 0.7371 | 0.7386 ± 0.0096 |
| A25 | 22.7500 ± 9.8970 | 0.5867 ± 0.0728 |
| A50 | 6.4979 ± 4.6193 | 0.8461 ± 0.1202 |
| A75 | 0.9919 ± 1.1739 | 1.2232 ± 0.2004 |
| 3L A75 1 ton | 5.6600 ± 7.0978 | 0.8921 ± 0.2156 |
| 3L A75 2 ton | 0.1254 ± 0.2233 | 1.5254 ± 0.0280 |
| 3L A75 3 ton | 0.5132 ± 1.1243 | 1.2909 ± 0.3528 |
| 3L A75 4 ton | 0.5214 ± 1.2590 | 1.2925 ± 0.3914 |
| 3L A75 40-mg | 2.8337 ± 3.1648 | 0.9608 ± 0.1820 |
| 3L A75 80-mg | 0.1254 ± 0.2233 | 1.5254 ± 0.0280 |
| 3L A75 100-mg | 0.1793 ± 0.5857 | 1.5157 ± 0.5311 |
| 3L A75 120-mg | 0.2879 ± 0.7805 | 1.4594 ± 0.4525 |
| A75 25 rpm | 23.7881 ± 8.2748 | 0.6057 ± 0.0619 |
| A75 50 rpm | 16.2166 ± 8.6058 | 0.6966 ± 0.0939 |
| A75 100 rpm | 0.9919 ± 1.1739 | 1.2232 ± 0.2004 |
| A75 150 rpm | 12.2281 ± 5.5749 | 0.8007 ± 0.0799 |
| 3L A75 25 rpm | 1.9565 ± 2.2003 | 1.0451 ± 0.1821 |
| 3L A75 50 rpm | 0.4155 ± 0.3639 | 1.3124 ± 0.1411 |
| 3L A75 100 rpm | 0.1254 ± 0.2233 | 1.5254 ± 0.0280 |
| 3L A75 150 rpm | 3.7696 ± 5.4134 | 1.1193 ± 0.2760 |
| 3L B75 | 7.9752 ± 3.1449 | 0.7780 ± 0.0654 |
| 3L C75 | 4.4306 ± 5.0314 | 1.2741 ± 0.2501 |
| 3L D75 | 9.6949 ± 4.5006 | 0.7241 ± 0.0759 |
| 3L E75 | 20.3293 ± 5.1237 | 0.5709 ± 0.0401 |
| 3L A55B20 | 7.9735 ± 3.1452 | 0.7781 ± 0.6550 |
| 3L A55C20 | 4.4306 ± 5.0313 | 1.2700 ± 0.2506 |
| 3L A55D20 | 9.6971 ± 4.5026 | 0.7241 ± 0.0759 |
| 3L A55F20 | 0.4039 ± 2.0426 | 1.5523 ± 0.9245 |
| 3L A55G20 | 3.1306 ± 3.5977 | 0.9442 ± 0.1862 |
| 3L A55H20 | 54.1671 ± 18.1073 | 0.5303 ± 0.6681 |
3L represents the three-layered tablet
aThe dissolution test performed in HCl buffer pH 1.2
The r2 and MSC from curve fitting of several single-layered tablets (A0a, A25a, A50a, A25, A75 25 rpm, A75 50 rpm) to first order kinetic were obviously higher (r2 = 0.9785–0.9990 and MSC = 3.57–6.62) than those of zero order and Higuchi’s kinetics. The n values from fitting to power law equation were in the range of 0.5658 to 0.6966. This indicated that the drug release from these tablets was by anomalous (non-Fickian) mechanisms (37,38) with an initially high release rate followed by a rapidly declining rate. Theophylline was reported to be released from matrix comprising wax combined with methylcellulose by diffusion mechanism of first order kinetic (39). A shift of drug release both in acidic medium and pH change to zero order kinetic occurred as the amount of lactose was increased.
By comparison, dissolution profiles of three-layered tablets mostly provided better fit to zero order kinetic than first order and Higuchi’s equation due to higher r2 and MSC values, suggesting that the developed three-layered tablets showed zero-order or Case II release. The values of the kinetic constant (k) were in accordance with the values of n, the diffusional exponent; with k having lower values when the transport mechanism was Case II and higher values for formulations that released the drug by Fickian diffusion (38,40). However, the dissolution profiles of some three-layered tablets could be best fitted with first order equation, especially in case of 3L B75, 3LD75, 3L E75, 3L A55B20, 3L A55D20 and 3L A55H20. Gel forming and erosion alterations of lateral surface and two laminated surfaces of three-layered tablets due to the addition of excipient with different aqueous solubility could change the drug diffusion from these controlled release devices. These indicated that the release of propranolol HCl from these tablets was by anomalous (non-Fickian) mechanisms. Therefore, type of diluent could affect the drug release mechanism of these three-layered tablets. Although, some investigations reported that the different excipient types had no influence on the release mechanism of drug from simple matrix tablet (17,41), such result was different in the case of the obtained layered tablets.
CONCLUSIONS
Increasing the amount of lactose could diminish the pH sensitive release behavior of chitosan–xanthan gum single layer matrix tablets. The amount of drug loading did not affect the drug release from three-layered tablet comprising chitosan–xanthan gum. The drug release from three-layered tablet was influenced by hydrodynamic force and alteration the matrix composition. The release rate was increased in correspond to the increasing in stirring rate. An increase in stirring rate was a corresponding increasing in the release rate. Incorporation of soluble diluents in core or barrier could enhance the drug release. By comparison, most dissolution profiles of three-layered tablets could provide better fit to zero order kinetic than first order kinetic and Higuchi’s equation.
Acknowledgements
The work was supported by Thailand Research Fund (Grant no. MRG4780076). This research work was kindly supported by the Faculty of Pharmacy, Silpakorn University. We appreciate Dr. Nalinee Poolsup, Dr. Somlak Kongmuang and Dr. Wisit Tangkeangsirisin for their invaluable comments during the preparation of the manuscript.
References
- 1.Phaechamud T., Ritthidej G. C. Sustained-release from layered matrix system comprising chitosan and xanthan gum. Drug Dev. Ind. Pharm. 2007;33:595–605. doi: 10.1080/03639040601015521. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda M., Peppas N. A., McGinity J. W. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int. J. Pharm. 2006;310:90–100. doi: 10.1016/j.ijpharm.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 3.Chidambaram N., Porter W., Flood K., Qiu Y. Formulation and characterization of new layered diffusional matrices for zero-order sustained release. J. Control Release. 1998;52:149–158. doi: 10.1016/S0168-3659(97)00207-1. [DOI] [PubMed] [Google Scholar]
- 4.Maggi L., Bruni R., Conte U. High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms. Int. J. Pharm. 2000;195:229–238. doi: 10.1016/S0378-5173(99)00402-0. [DOI] [PubMed] [Google Scholar]
- 5.Conte U., Maggi L. Modulation of the dissolution profiles from GeomatrixÒ multi-layer matrix tablets containing drugs of different solubility. Biomater. 1996;17:889–896. doi: 10.1016/0142-9612(96)83284-4. [DOI] [PubMed] [Google Scholar]
- 6.Ford J. L., Rubinstein H. M., McCaul F., Hogan J. E., Edgar P. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. IL. Farmaco. 1987;40:223–234. [Google Scholar]
- 7.Lotfipour F., Nokhodchi A., Saeedi M., Norouzi M., et al. The effect of hydrophilic and lipophilic polymers and fillers on the release rate of atenolol from HPMC matrices. IL. Farmaco. 2004;59:819–825. doi: 10.1016/j.farmac.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Yang L., Fassihi R. Examination of drug solubility, polymer types, hydrodynamics and loading dose on drug release behavior from a triple-layer asymmetric configuration delivery system. Int. J. Pharm. 1997;155:219–229. doi: 10.1016/S0378-5173(97)00164-6. [DOI] [Google Scholar]
- 9.Wu Y., Kildsig D. O., Ghaly E. S. Effect of hydrodynamic environment on tablets dissolution rate. Pharm. Dev. Tech. 2004;9(1):25–37. doi: 10.1081/PDT-120027415. [DOI] [PubMed] [Google Scholar]
- 10.MicroMath Scientist Handbook Rev. 7EEF, MicroMath: Salt Lake City, 1995, p. 467.
- 11.Perissutti B., Michael J., Podczeck F., Rubessa F. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid release dosage form. Eur. J. Pharm. Biopharm. 2002;53:125–132. doi: 10.1016/S0939-6411(01)00209-0. [DOI] [PubMed] [Google Scholar]
- 12.Perissutti B., Rubessa F., Moneghini M., Voinovich D. Formulation design of carbamazepine fast-release tablets prepared by melt granulation technique. Int. J. Pharm. 2003;256:53–63. doi: 10.1016/S0378-5173(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q.-R., Choi Y.-W., Cui J.-H., Lee B.-J. Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J. Control Release. 2005;108:351–361. doi: 10.1016/j.jconrel.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Khanz H., Brun V. L., Wagner T. Development of a multiparticulate extended release formulation for ZK 811752, a weakly basic drug. Int. J. Pharm. 2005;299:84–91. doi: 10.1016/j.ijpharm.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Xu G., Sunada H. Influence of formulation change on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Chem. Pharm. Bull. 1995;43:483–484. doi: 10.1248/cpb.43.483. [DOI] [PubMed] [Google Scholar]
- 16.Gao P., Skoug J. W., Nixon P. R., Ju T. R., Stemm N. L., Sung K. C. Swelling of the influence of formulation variable on matrix performance and drug release. J. Pharm. Sci. 1996;85:732–740. doi: 10.1021/js9504595. [DOI] [PubMed] [Google Scholar]
- 17.Williams R. O., Reynolds T. D., Cabelka T. D., Sykora M. A., Mahaguna V. Investigation of excipient type and level on drug release from controlled release tablets containing HPMC. Pharm. Dev. Tech. 2002;7(2):181–193. doi: 10.1081/PDT-120003486. [DOI] [PubMed] [Google Scholar]
- 18.Dumitriu S., Magny P., Montane D., Vidal P. F., Chornet E. Polyionic hydrogels obtained by complexation between xanthan and chitosan: their properties as supports for enzyme immobilization. J. Bioact. Compat. Polym. 1994;9:184–209. doi: 10.1177/088391159400900205. [DOI] [Google Scholar]
- 19.Dumitriu S., Chornet E. Inclusion and release of proteins from polysaccharide-based polyion complexes. Adv. Drug Del. Rev. 1998;31:223–246. doi: 10.1016/S0169-409X(97)00120-8. [DOI] [PubMed] [Google Scholar]
- 20.Riis T., Bauer-Brandl A., Wagner T., Kranz H. pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. Eur. J. Pharm. Biopharm. 2007;65:78–84. doi: 10.1016/j.ejpb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Velasco M. V., Ford J. L., Rowe P., Rajabi-Siahboomi A.R. Influence of drug:hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J. Control Release. 1999;57:75–85. doi: 10.1016/S0168-3659(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 22.Ebube N. K., Jones A. B. Sustained release of acetaminophen from a heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymers. Int. J. Pharm. 2004;272:19–27. doi: 10.1016/j.ijpharm.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Cartilier L., Hartman N. G. Substituted amylose as a matrix for sustained-drug release: a biodegradation study. Int. J. Pharm. 2001;222(2):183–189. doi: 10.1016/S0378-5173(01)00694-9. [DOI] [PubMed] [Google Scholar]
- 24.Conte U., Maggi L., Colomba P., La Manna A. Multi-layered hydrophilic matrices as constant release devices (Geomatrixä Systems) J. Control Release. 1993;26:39–47. doi: 10.1016/0168-3659(93)90207-L. [DOI] [Google Scholar]
- 25.El-Gazayerly O. N. Release of pentoxifylline from xanthan matrix. Drug Dev. Ind. Pharm. 2003;29(2):241–246. doi: 10.1081/DDC-120016732. [DOI] [PubMed] [Google Scholar]
- 26.Kim H., Fassihi R. Application of binary polymer system in drug release rate modulation. 2. influence of formulation variables and hydrodynamic conditions on release kinetics. J. Pharm. Sci. 1997;86:323–328. doi: 10.1021/js960307p. [DOI] [PubMed] [Google Scholar]
- 27.Zuleger S., Fassihi R., Lippold B. C. Polymer particles erosion controlling drug release. II. Swelling investigations to clarify the release mechanism. Int. J. Pharm. 2002;247:23–37. doi: 10.1016/S0378-5173(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 28.Herman J., Remon J. P. Modified starches as hydrophilic matrices for controlled oral delivery. II. In vitro drug release evaluation of thermally modified starches. Int. J. Pharm. 1989;56:65–70. doi: 10.1016/0378-5173(89)90061-6. [DOI] [Google Scholar]
- 29.Durig T., Fassihi R. Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substance and excipients on gel dynamics and release kinetics. J. Control Release. 2002;80:45–56. doi: 10.1016/S0168-3659(01)00546-6. [DOI] [PubMed] [Google Scholar]
- 30.Ho H.-O., Wang H.-Y., Sheu M.-T. The evaluation of granulated excipients as matrix material for controlled delivery of captopril. J. Control Release. 1997;49:243–251. doi: 10.1016/S0168-3659(97)00093-X. [DOI] [Google Scholar]
- 31.Pillay V., Fassihi R. A novel approach for constant rate delivery of highly soluble bioactives from a simple monolithic system. J. Control Release. 2000;67:67–78. doi: 10.1016/S0168-3659(00)00193-0. [DOI] [PubMed] [Google Scholar]
- 32.Pillay V., Fassihi R. In situ electrolyte interactions in a disk-compressed configuration system for up-curving and constant drug delivery. J. Control Release. 2000;67:55–65. doi: 10.1016/S0168-3659(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 33.Levy G., Schwarz T. W. The effect of certain additives on the gel point of methylcellulose. J. Am. Pharm. Assoc. 1958;47:44–46. doi: 10.1002/jps.3030470113. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J. Appl. Polym. Sci. 1979;24:1073–1087. doi: 10.1002/app.1979.070240420. [DOI] [Google Scholar]
- 35.Talukdar M. M., Vinckier I., Moldenaers P., Kinget R. Rheological characterization of xanthan gum and hydroxypropylmethyl cellulose with respect to controlled-release drug delivery. J. Pharm. Sci. 1996;85:537–540. doi: 10.1021/js950476u. [DOI] [PubMed] [Google Scholar]
- 36.Khan G. M., Jiabi Z. Formulation and in vitro evaluation of ibuprofen-carbopoÒ 974P-NF controlled release matrix tablets. III: influence of co-excipients on release rate of the drug. J. Control Release. 1998;54:185–190. doi: 10.1016/S0168-3659(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 37.Peppas N. A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985;60(4):110–111. [PubMed] [Google Scholar]
- 38.Nerurkar J., Jun H. W., Price J. C., Park M. O. Controlled-release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rates. Eur. J. Pharm. Biopharm. 2005;61:56–68. doi: 10.1016/j.ejpb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Venkatesh S. S., Hiremath D. Formulation and evaluation of sustained release dosage form of theophylline using a combined hydrophobic and hydrophilic matrix. Indian J. Pharm. Sci. 2000;62(1):33–36. [Google Scholar]
- 40.Ritger P. L., Peppas N. A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 41.Akbari J., Nokhodchi A., Farid D., Siahi-Shadbad M. R., Saeedi M. Development and evaluation of buccoadhesive propranolol hydrochloride tablet formulations: effect of fillers. Farmaco. 2004;59(2):155–151. doi: 10.1016/j.farmac.2003.11.011. [DOI] [PubMed] [Google Scholar]