Abstract
The purpose of the present investigation is to formulate and evaluate proniosomal transdermal carrier systems for flurbiprofen. Proniosomes were prepared using various non-ionic surfactants, namely span 20 (Sp 20), span 40 (Sp 40), span 60 (Sp 60) and span 80 (Sp 80) without and with cholesterol at percentages ranging from 0% to 50%. The effect of surfactant type and cholesterol content on drug release was investigated. Drug release was tested by diffusion through cellophane membrane and rabbit skin. Drug release from the prepared systems was compared to that from flurbiprofen suspensions in distilled water and HPMC (hydroxypropylmethylcellulose) gels. In case of Sp 20 and Sp 80, the added amount of cholesterol affected the preparation type to be either proniosomal alcoholic solutions or liquid crystalline gel systems. On the other hand, both Sp 40 and Sp 60 produced gel systems in presence or absence of cholesterol. Microscopic observations showed that either proniosomal solutions or gel formulations immediately converted to niosomal dispersions upon hydration. Due to the skin permeation barrier, rabbit skin showed lower drug diffusion rates compared to cellophane membrane. The proniosomal composition controlled drug diffusion rates to be either faster or slower than the prepared flurbiprofen suspensions in HPMC gels or distilled water, respectively. In conclusion, this study demonstrated the possibility of using proniosomal formulations for transdermal drug delivery.
Key words: cholesterol, flurbiprofen, gel, liquid crystal, non-ionic surfactant, proniosomes
INTRODUCTION
The self-assembly of non-ionic amphiphiles in aqueous media resulting in closed bilayer structures leads to the formation of niosomal vesicles or niosomes. Niosomal vesicles are analogous to liposomes (phospholipid vesicles) and serve as drug carriers because they can encapsulate both hydrophilic and lipophilic substrates. Among all routes of administration, the enhanced transdermal delivery of niosome encapsulated drugs was considered (1–3). Uchegbu and Vyas, reported that transdermal drug delivery with niosomes appear promising for hydrophobic and amphiphilic drug molecules and require the dose to be applied in high concentration and within niosomes prepared from low phase transition surfactant mixtures (4). The vesicular systems can overcome the permeation barrier of the skin and act as penetration enhancers for the drug (5). Moreover, these systems can be used as vehicles for controlled transdermal drug delivery (6). Niosomes are chemically stable, easy to store and of lower cost compared to phospholipid liposomes (7). However, aqueous suspensions of niosomes may exhibit physical instability like liposomes. Aggregation and fusion of vesicles and leaking or hydrolysis of the encapsulated drug might contribute in decreasing niosomal shelf life. In addition, for transdermal application, niosomal vesicles should be included into polymeric matrix like methylcellulose gels. However, the use of polymeric matrix in the formulation may affect drug penetration and niosome integrity (1). This problem in addition to the physical instability of the niosomal dispersion can be circumvented by the formulation of liquid crystalline compact proniosomal gels or alcoholic solutions of the non ionic surfactant. Proniosomal gels and solutions are of great stability due to very little water content. Gels or alcoholic solutions of nonionic surfactants can transform into niosomal vesicles immediately upon hydration, hence, called proniosomes. The great advantage offered by proniosomes is their ease of use and their hydration is much easier than the long shaking process required to hydrate surfactants in the conventional dry film method (5). Furthermore, unacceptable solvents are avoided in proniosomal formulations. The systems may be directly formulated into transdermal patches and doesn’t require the dispersion of vesicles into polymeric matrix (8).
Flurbiprofen is a derivative of phenylalkanoic acid, a nonsteroidal anti-inflammatory drug (NSAID) related to ibuprofen in structure. It has an intrinsic solubility of 5.0 × 10−5 M. The commercial dosage forms of flurbiprofen are tablets,sustained release capsules, and eye drops (9). It is used in the treatment of gout, rheumatoid arthritis, osteoarthritis and other rheumatic disorders. Administration of flurbiprofen via the skin could have benefits over oral administration, since it is a non-invasive administration (convenient and safe) and is suitable to people who can’t use the oral route due to vomiting or unconsciousness. The transdermal route also can avoid gastrointestinal (GI) incompatibility, variable GI absorption, first pass metabolism and the GI side effects of the drug. Moreover, it can reduce the frequency of administration and improve patient compliance. Transdermal dosage forms of flurbiprofen such as gels, ointments and creams have been intensively studied for this purpose (10–12).
The aim of this study was to develop flurbiprofen proniosomal carrier systems using the common, non-irritant, safe and available non-ionic surfactants “Sp 20, Sp 40, Sp 60, and Sp 80” with and without cholesterol. The prepared systems hypothesized to have controlled release for flurbiprofen over extended period of time. To investigate the possibility of using proniosomal systems for transdermal delivery of drugs, in vitro release and permeation studies of flurbiprofen were tested and compared with some other suspension forms of the drug using cellophane membranes and rabbit skin. The effect of cholesterol on drug permeation was also evaluated.
MATERIALS AND METHODS
Flurbiprofen was a gift from Egyptian International pharmaceutical industries co. (E.I.P.I.Co), Egypt. Sorbitan monolaurate (Sp 20), sorbitan monopalmitate (Sp 40), sorbitan monostearate (Sp 60), sorbitan mono-oleate (Sp 80), sodium azide, and cholesterol (Chol; >99%) were purchased from Sigma chemical Co., St. Louis, MO, USA. Spectrapore® nitrocellulose membranes (MWCO 2,000–15,000) were obtained from Spectrapore Inc., NY, USA. All other chemicals and solvents were of analar grade and obtained from El-Nasr Company for pharmaceutical chemicals, Cairo, Egypt.
Preparation of Proniosomes
Proniosomes were prepared by the method reported by Vora et al., with some modifications (8). In glass vials accurately weighed amounts of the surface active agent were mixed with the appropriate amount of cholesterol to make 1 mmol total lipids. The amounts of cholesterol added as 10% increments varied from 0% to 60% of total lipids (Table I). Absolute ethanol (about 400 mg) was added to the surfactant or surfactant/cholesterol mixtures then vials were tightly sealed and warmed in water bath (55–60°C) for 5 min while shaking until complete dissolution of cholesterol and surfactant. To each of the formed transparent solutions, about 0.16 ml hot distilled water (55–60°C) was added while warming in the water bath for 3–5 min till a clear or translucent solution was produced. The mixtures were allowed to cool down at room temperature and observed for the formation of transparent solution, two phase liquids, translucent, transparent or white creamy proniosomal gel as shown in Table I. The obtained formulations were kept in the same closed glass vials in dark for further characterization.
Table I.
Composition and Appearance of Nonionic Surfactant/Cholesterol/Absolute Ethanol Mixtures
| Cholesterol (molar %) | Sp 20 | Sp 40 | Sp 60 | Span 80 |
|---|---|---|---|---|
| 0 | Transparent liquid (one phase) | White creamy gel | White creamy gel | Two phases liquid |
| 10 | Transparent liquid (one phase) | White creamy gel | White creamy gel | Two phases liquid |
| 20 | Transparent gel | White creamy gel | White creamy gel | Two phases liquid |
| 30 | Transparent gel | White creamy gel | White creamy gel | Translucent gel |
| 40 | Translucent gel | White creamy gel | White creamy gel | Translucent gel |
| 50 | Translucent gel | White creamy gel | White creamy gel | Translucent gel |
| 60 | Translucent gel | White creamy gel | White creamy gel | Translucent gel |
Flurbiprofen was added as 50 mg to the nonionic surfactant/cholesterol mixture and dissolved by the aid of absolute ethanol while warming at 50–60°C in water bath.
Microscopic Examination
Small amounts of the formed proniosomal gels were spread on a glass slide as a thin layer and examined for the gel structure and the presence of insoluble drug crystals using ordinary light microscope with varied magnification powers (×10 and ×40) (8). The proniosomal gels containing the drug were spread as thin layers on a glass slide with one drop of water excess and examined under microscope for niosomal vesicles formation and the presence of drug crystals (5). Photomicrographs were taken for either proniosomal gel or niosomes using Fujifilm digital camera.
Preparation of HPMC Gels Containing Flurbiprofen
Either aqueous or alcoholic gels (containing about 50% ethanol) of HPMC-400 cP are simply prepared as reported in Kibbe (13). For the preparation of aqueous gels, the weighed quantities of HPMC-400 cP were dispersed in distilled water and sufficient time (about 3 to 4 h) was allowed for complete hydration and gel formation. On the other hand, to prepare alcoholic gels about 50% of the distilled water was replaced with absolute ethanol. One gram of flurbiprofen was added and dispersed thoroughly. The concentration of HPMC-400 cP was 4% and the final concentration of flurbiprofen was adjusted to be 50 mg/g. The viscosity of the prepared gels was determined using Visco Star-R FUNGILAB viscometer. Spindle number 5 was used and the rotation speed of the spindle was adjusted at 2 rpm.
In Vitro Skin Permeation Study
Backing Membrane Reservoirs
Circular plastic holders of 2.9 cm inner diameter and 1 mm edge height were used as backing membrane reservoirs for proniosomal gels. Simply, the warm proniosomal solutions before gelling were poured into the plastic holders and covered immediately by small containers to avoid solvent loss. After cooling, the formed gels were used immediately for the release or skin permeation experiments. These holders provided even spread of the gel and fixed surface area (6.61 cm2) exposed to the membrane or the skin during the release procedures.
Preparation of Rabbit Skin
Albino male rabbits (2–2.5 kg) were obtained from Faculty of Veterinary Medicine, Zagazig University, animal breeding center, Egypt. Hair was removed from the abdominal skin with the aid of an electric animal clipper and shaver. Care was taken not to damage the skin surface. Rabbits were sacrificed by administration of excess chloroform inhalation and the abdominal skin of the rabbit was separated. The skin was stored at −20°C and used within three days for the permeation study. It has been reported that storage in the refrigerator keeps the metabolic activity of the skin (14). Before the permeation study, the skin was hydrated in phosphate buffer pH 7.4 (containing 0.02% sodium azide as a preservative) at 4°C over night and the adipose tissue layer of the skin was removed by rubbing with a cotton swab (15).
Permeation of Flurbiprofen from Different Formulations
The in-vitro permeation of flurbiprofen from different proniosomal preparations was determined by using a dissolution-dialysis apparatus the cell of which is developed in our laboratory. The dissolution cell consisted of a hollow glass cylinder (length 15 cm and internal diameter of 2.9 cm). The skin membranes were mounted, with the stratum corneum side towards the donor (drug loaded system) and the dermal side facing the receptor compartment which contained 100 ml phosphate buffer pH 7.4 and 0.02% sodium azide as preservative and maintained at 37 ± 0.1°C (16). This volume provides complete sink conditions for the drug as the saturated solubility of flurbiprofen was found to be 3.81 ± 0.11 mg/ml in this medium. Proniosomal solutions and drug suspension (50 mg/ml) were applied to the stratum corneum side, however, proniosomal gels and drug polymer gels were put into membrane holders and fixed to the glass tubes (previously described), then skin membranes used to cover the gel preparation with the stratum corneum side face. The tubes were attached to the dissolution apparatus with Parafilm® sealing film (American National Can Company, Chicago, IL, USA) to avoid alcohol or water evaporation and allowed to stir at 100 rpm. At 0.25, 0.5, 0.45, 1, 2, 3, 4, 5, 6, 8, 10, and 24 h after starting the experiment, 3 ml aliquots were sampled from the receptor compartment with the fresh buffer replacement. Samples were analyzed spectrophotometrically at 247 nm (Schimadzu U.V.-1201, Cat NO. 206-62409, Schimadzu Corporation, Japan) using samples collected from permeation of drug free systems as a blank. The obtained absorbance was interpreted using standard calibration curve of flurbiprofen in Sorensen’s phosphate buffer (pH 7.4) at 247 nm and drug concentration range from 2 to 14 μg/ml. Each experiment was carried out for 24 h and repeated in triplicate. In vitro permeation rate studies such as steady state transdermal flux (SSTF), permeability coefficient (PC), and enhancement ratio (ER) for transport of flurbiprofen across rabbit skin were estimated for different formulations and effect of variation in composition (e.g. spans and cholesterol) on release rate was studied.
Calculations for the in vitro permeation rate studies are as follows (8).
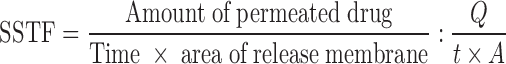 |
 |
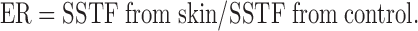 |
Release of Flurbiprofen from Semipermeable Cellophane Membranes
To compare the effect of membrane on drug diffusion semipermeable cellophane membranes were used in place of rabbit skin and the experiment run as previously mentioned. The data were reported as mean ± SD (n = 3) and statistical analysis of the data were carried out using one way ANOVA followed by LSD test at a level of significant of P < 0.05.
RESULTS AND DISCUSSION
Proniosomal gel was prepared by first forming the sol phase at high temperature (60°C). Since the solvent (absolute ethanol) amount was small and the formation of micelles is not possible into this solvent (17), the addition of small amount of water could favor the self assembly of the surfactant into w/o microemulsion sol phase, where the aqueous droplets bound by surfactant interfacial films were dispersed in the continuous solvent phase (18–19). Cooling results in a decrease in the solubility of the sorbitan mono-ester and/or cholesterol (gelators) in the solvent and lowers solvent-gelator affinities due to the limited solvent system present. The formed gel structure suggested to be a lamellar micellar model (amphiphilic system) comprising double layers of oriented molecules placed end to end and tail to tail with water present as droplets or sheets of water molecules between the hydrophilic residues of the surfactant layers. Both Sp 20 and Sp 80 which have the lowest transition temperatures (13) (16°C and −12°C, respectively) are liquids at room temperature and cannot form gels at lower concentrations of cholesterol (Table I). Gel formation will start at 20% and 30% (molar percent) of cholesterol for Sp 20 and Sp 80, respectively. Since Sp 80 is more hydrophobic (HLB = 4.3) than Sp 20 (HLBB = 8.6), it produced two phases proniosomal liquids at cholesterol concentrations below 30%. On the other hand, Sp 40 (Tc = 42°C) and Sp 60 (Tc = 53°C) produced white creamy gels in the presence or absence of cholesterol as they have high transition temperatures and are solids at room temperature (13), so they act as gelators by themselves. The formed proniosomal gels were found to be thermo-reversible.
The complete hydration of proniosomal gels was found to take long time ranging from one hour to two hours at room temperature with continuous stirring or vortexing. This could be explained depending on the concept of coacervation-phase separation as the solvent “absolute ethanol” is hydrophilic and consequently coacervation is delayed at room temperature. Warming the gel with excess water at 60°C for 10 min had accelerated the transformation into niosomal structures.
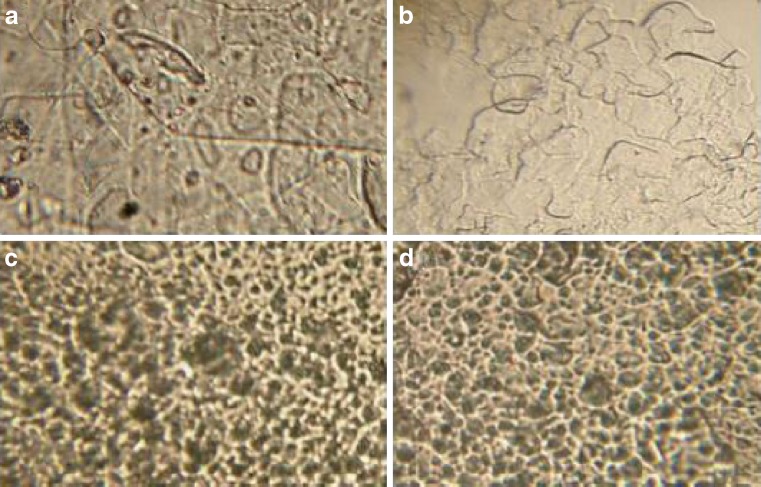
The addition of flurbiprofen didn’t affect the final appearance of the formed formulation which indicates the complete dissolution of the drug into the final preparation. Figure 1 shows the different gel structures according to the surfactant type. From a and b photomicrographs, gels of Sp 20 and Sp 80 consist of twisted matted lamellar strands with transparent appearance and no drug precipitates, where c and d show that Sp 40 and Sp 60 gels are formed of floccules of small tubular and vesculating particles which have creamy opaque appearance and also no drug precipitates. The units of the gel are often bound together by van der Waals forces so as to form crystalline regions throughout the entire system (20). Examination of different gels of various surfactants with and without cholesterol revealed that cholesterol content didn’t affect the overall gel structure “data not shown”.
Fig. 1.
Photomicrographs of proniosomal gels of different spans (×40). a Sp 20/20% cholesterol, b Sp 80/30% cholesterol, c Sp 40/0% cholesterol, and d Sp 60/0% cholesterol
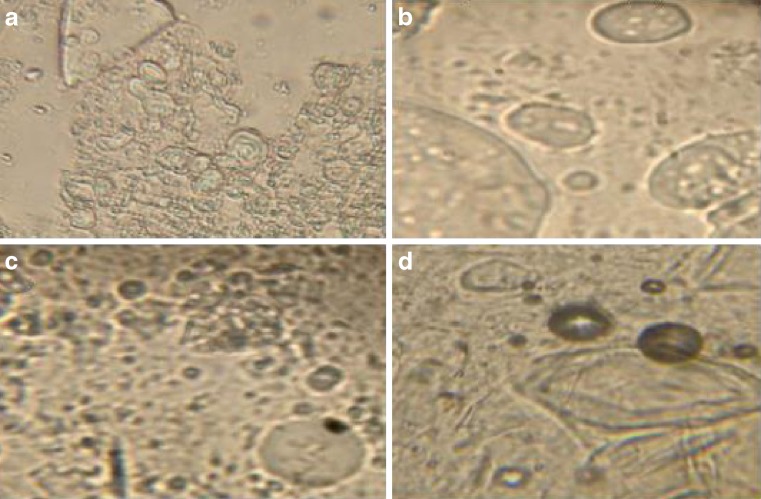
All span surfactants give rise to randomly scattered spherical structures comprising multilamellar and multivesicular vesicles when hydrated (Fig. 2). The consequences of transformation from gel to niosomal structures can be noted through the remaining swelled gel fractions (Fig. 2: a,b, and c) or through clearly defined striations which indicate bilayer structure in case of Sp 60 photomicrograph (Fig. 2: d).
Fig. 2.
Photomicrograph of hydrated proniosomes of different spans (×40) showing swelled gel fragments and varied size niosomes. a Sp 20/20% cholesterol, b Sp 80/30% cholesterol, c Sp 40/0% cholesterol, and d Sp 60/0% cholesterol
In Vitro Rabbit Skin Permeation Study
Although animal skin has different (generally higher) drug permeability compared to human skin, skin of rodents (mice, rats, rabbits and guinea pigs) is the most commonly used in in vitro and in vivo percutaneous permeation studies due to its availability. The advantages of these animals are their small size, uncomplicated handling and relatively low cost (21). Taking into consideration these advantages, rabbit skin was selected for the permeability studies.
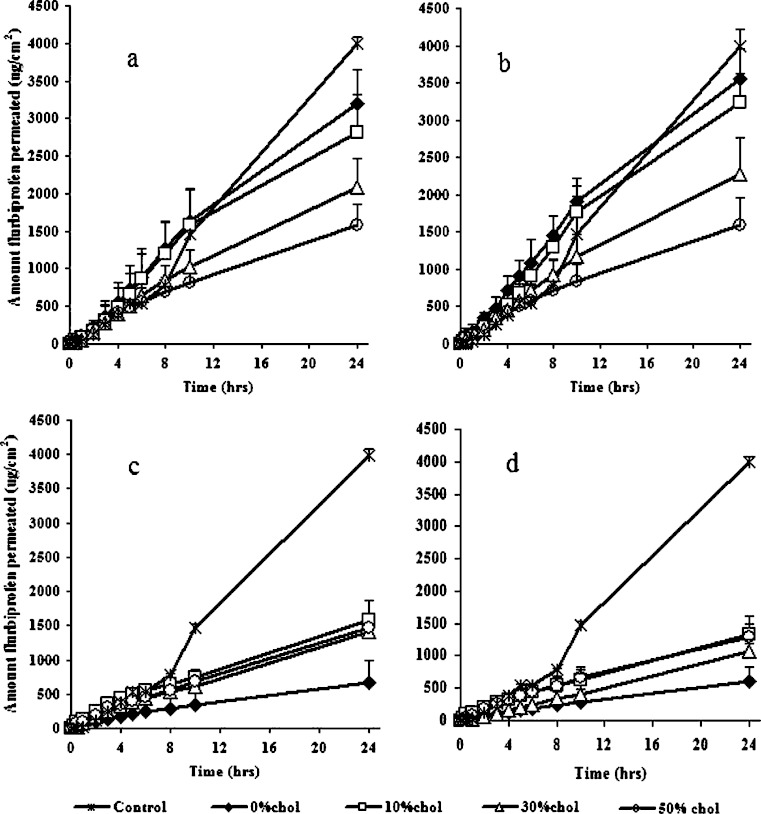
Figure 3 shows the cumulative amounts of flurbiprofen transferred from proniosomal formulations to the receptor compartment (phosphate buffer pH 7.4). Throughout the experiment period, proniosomal liquids of both Sp 20 and Sp 80 showed a larger amount of flurbiprofen penetration than the proniosomal gel formulations of both Sp 40 and Sp 60. After 24 h about 3,200, 3,562, 663, and 614.84 μg/cm2 of flurbiprofen were permeated through rabbit skin from Sp 20, Sp 80, Sp 40, and Sp 60 cholesterol free proniosomal formulations, respectively. The steady state transdermal fluxes (SSTF) of flurbiprofen from cholesterol free proniosomal liquids of Sp 20 and Sp 80 were more than five times those of both Sp 40 and Sp 60 cholesterol free gels (Table II). Statistical analysis showed a significant difference between SSTF of cholesterol free proniosomes of Sp 80 and Sp 20 (P = 0.037) whereas, no significant difference could be existed between that of Sp 40 and Sp 60 cholesterol free formulations (P = 0.962). Liquid proniosomes of Sp 20 and Sp 80 containing 10% cholesterol also showed higher permeability than that of Sp 40 and Sp 60 proniosomal gels. The SSTF of flurbiprofen from Sp 20/10% cholesterol and Sp 80/10% cholesterol proniosomes was more than two times that of Sp 40/10% cholesterol and Sp 60/10% cholesterol (Table II). The reduced permeation of flurbiprofen from proniosomal gels of Sp 40 and Sp 60 was primarily attributed to their high transition temperatures which made them in a highly ordered gel state at the permeation temperature (37°C) (22–23). On the other hand, the lower transition temperatures of Sp 20 and Sp 80 made them in the disordered liquid crystalline state and completely fluid, hence, they are more permeable to the drug at 37°C. Moreover, the presence of unsaturated double bond in the oleate side chain of span 80 was responsible for the significant enhancement of flurbiprofen permeation. The packing nature of unsaturated fatty acids changes the stratum corneum lipid structure and facilitates the drug permeability (24). Topically applied surfactant vesicles may extract the lipid from the skin or disrupt the order within and between the corneocyte upon binding to the keratin filament; hence increase drug permeability across skin (25).
Fig. 3.
Effect of cholesterol on flurbiprofen permeability from proniosomes of different spans across rabbit skin. a Sp 20, b Sp 80, c Sp 40, and d Sp 60. Each point represents the mean ± SD (n = 3)
Table II.
Steady State Transdermal Flux (SSTF), Permeability Coefficient, Correlation Coefficient of Straight Line and Enhancement Ratio of Flurbiprofen Transport Across Rabbit Skin After 24 h
| Formulation | SSTF (μg/cm2 h) | Permeability coefficient (cm2 h−1) | Correlation coefficient (R) | Enhancement ratio (ER)a |
|---|---|---|---|---|
| Drug suspension in distilled water (control) | 160.85 ± 9.55 | 0.281 ± 0.017 | 0.9883 | 1 |
| Sp 20/0% chol | 135.27 ± 18.61 | 0.235 ± 0.032 | 0.9943 | 0.841 |
| Sp 20/10% chol | 117.04 ± 18.8 | 0.203 ± 0.033 | 0.9872 | 0.728 |
| Sp 20/30% chol | 95.36 ± 9.84 | 0.165 ± 0.0171 | 0.9936 | 0.593 |
| Sp 20/50% chol | 65.93 ± 11.61 | 0.114 ± 0.02 | 0.9872 | 0.410 |
| Sp 40/0% chol | 26.49 ± 14.08 | 0.046 ± 0.024 | 0.9857 | 0.165 |
| Sp 40/10% chol | 58.62 ± 11.83 | 0.102 ± 0.021 | 0.9884 | 0.364 |
| Sp 40/30% chol | 58.64 ± 3.22 | 0.102 ± 0.006 | 0.9956 | 0.365 |
| Sp 40/50% chol | 61.21 ± 7.48 | 0.106 ± 0.013 | 0.9946 | 0.381 |
| Sp 60/0% chol | 25.97 ± 4.86 | 0.045 ± 0.01 | 0.9962 | 0.162 |
| Sp 60/10% chol | 48.05 ± 11.6 | 0.083 ± 0.02 | 0.9939 | 0.299 |
| Sp 60/30% chol | 47.20 ± 4.74 | 0.082 ± 0.01 | 0.9874 | 0.287 |
| Sp 60/50% chol | 53.78 ± 8.73 | 0.093 ± 0.015 | 0.9890 | 0.334 |
| Sp 80/0% chol | 158.43 ± 16.21 | 0.280 ± 0.028 | 0.9924 | 0.985 |
| Sp 80/10% chol | 134.95 ± 14.45 | 0.234 ± 0.025 | 0.9909 | 0.839 |
| Sp 80/30% chol | 95.07 ± 20.56 | 0.165 ± 0.036 | 0.9926 | 0.591 |
| Sp 80/50% chol | 66.35 ± 15.51 | 0.115 ± 0.071 | 0.9868 | 0.413 |
| HPMC gel | 53.27 ± 10.17 | 0.092 ± 0.02 | 0.9932 | 0.331 |
| HPMC alcoholic gel | 66.35 ± 15.51 | 0.115 ± 0.03 | 0.9930 | 0.413 |
Each result is the mean value ± SD (n = 3).
aER = SSTF of formulation/SSTF of control
The permeability of flurbiprofen through rabbit skin was found to be greatly affected by the amount of cholesterol incorporated into proniosomes. Cholesterol was significantly enhancing flurbiprofen permeability from proniosomal formulations of both Sp 40 and Sp 60 (P < 0.05). In contrast it significantly retarded the drug permeability from proniosomal systems of both Sp 20 and Sp 80. It is argued that addition of cholesterol appeared to disrupt the ordered array of the hydrocarbon chains in the gel phase (26). Below the surfactant transition temperature, addition of cholesterol made the membrane less ordered, while above the surfactant transition temperature it made the membrane more ordered (27). From the obtained results by increasing cholesterol amounts from 0% to 50% into either Sp 20 or Sp 80 proniosomes the amount drug permeated after 24 h reduced from 3,246 to 1,582 μg/cm2 or from 3,562 to about 1,592 μg/cm2, respectively (Fig. 3). Statistical analysis showed no significant decrease in SSTF by incorporation of 10% cholesterol in proniosomal liquids of Sp 20 (P = 0.09), however, a highly significant decrease in SSTF resulted as gelling occurred by using 30% and 50% cholesterol (P = 0.001 and P = 0.000, respectively). Moreover, a significant decrease in SSTF of flurbiprofen could exist among all Sp 80 proniosomal systems as cholesterol content increased from 0% to 50% (P < 0.05). Comparing Sp 20 and Sp 80 proniosomes, the later showed higher drug permeability and significant increase (P < 0.05) in flurbiprofen SSTF except for formulae containing 30% and 50% cholesterol (P > 0.05). The effect of cholesterol on the amount flurbiprofen permeated from both Sp 40 and Sp 60 proniosomes was opposite to that in case of Sp 20 and Sp 80. The amount drug permeated through rabbit skin had increased from 663 to 1,578 μg/cm2 (Sp 40 proniosomes) and from 614 to 1,338 μg/cm2 (Sp 60 proniosomes) as the cholesterol amount was increased from 0% to 10%. Further increase in cholesterol molar percent (to 30% and 50%) showed no significant decrease in flurbiprofen permeability and no significant difference in SSTF when compared to formulations containing 10% cholesterol (P > 0.05). The SSTF of flurbiprofen from Sp 40 and Sp 60 proniosomal gels containing cholesterol was about twice or more than twice the SSTF of flurbiprofen from cholesterol free preparations of the same surfactants (Table II). Moreover, there was no significant difference in SSTF of flurbiprofen between Sp 40 and Sp 60 proniosomes when having 10% or more cholesterol (P > 0.05). At 30% of cholesterol, proniosomal gel of Sp 20 showed no significant SSTF of flurbiprofen compared to Sp 80 formula (P = 0.978). On the other hand, results revealed that both systems gave significant increase in SSTF of flurbiprofen (more than 1.5 times) when compared to Sp 40 and Sp 60 systems having 30% cholesterol (P < 0.05). When cholesterol molar percent was increased to 50% of the proniosomal formulations, the difference in the amounts of flurbiprofen permeated as well as the change in the SSTF was not significant whatever the surfactant used was of high or low transition temperature (P > 0.05). The results are in accordance with the reported by Vanhal et al. (3) who suggested that reducing cholesterol content caused an increase in the permeation of estradiol. However, other reports suggested that increasing cholesterol content in the vesicles did not affect the transdermal delivery of ketorolac tromethamine and estradiol drugs (23,28). These results may indicate different mechanisms of drug transport across the skin depending on the composition of proniosomes and the drug used.
Proniosomes in Comparison with Other Non Vesicular Systems
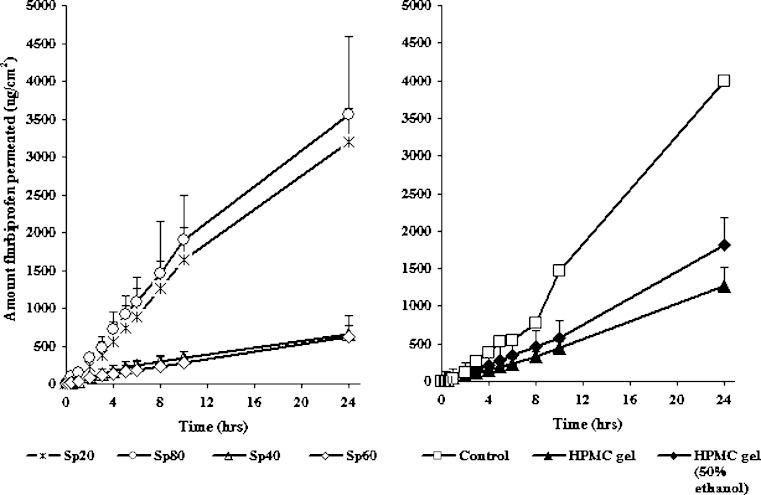
Figure 4 shows the amount of drug permeated through rabbit skin from the suspensions of flurbiprofen in distilled water (the control) and HPMC gels compared to cholesterol free proniosomal systems. The cholesterol free proniosomal systems were selected because they displayed the highest (Sp 20 and Sp 80 systems) and the lowest (Sp 40 and Sp 60 systems) permeation to flurbiprofen among all proniosomes tested. Results showed controlled drug delivery for flurbiprofen when all formulae compared to the control. This is clear from the SSTF, PC, and the enhancement ratios listed in Table II. Statistical analysis showed no significant differences (P > 0.05) in flurbiprofen permeation and SSTF between the control and proniosomal liquid formulations of Sp 20 and Sp 80. Moreover, the decrease in drug permeability and SSTF was highly significant between proniosomes of Sp 40, Sp 60, gels of Sp 20 and Sp 80, HPMC gels, and the control (P < 0.05). The increase in flurbiprofen permeability from liquid proniosomal formulations is discussed before. However, in case of proniosomal gels, the semisolid state and the lamellar structures of proniosomes could hinder or decrease drug permeability through lipid bilayer membranes (29). On the other hand, the lower drug transport through polymeric gels could be due to a combination of diffusion through the matrix, matrix relaxation due to water absorption and a little erosion (30).
Fig. 4.
In vitro permeation of flurbiprofen from different vehicles at 0% cholesterol. Each point represents the mean ± SD (n = 3)
The SSTF of flurbiprofen increased due to ethanol addition into HPMC gel (Table II). Ethanol may cause the reduction of lipid polar head interactions or may disorder liquid–crystalline phases within the membrane, thereby resulting in increased skin permeation (30–32). However, statistical analysis revealed the enhancement of flurbiprofen permeability from alcoholic HPMC gel compared to aqueous gel was not significant (P = 0.318). This could be due to the higher determined viscosity of ethanolic HPMC gel compared to that of aqueous gel (1,046.35 ± 179.1 and 716.9 ± 41 poise, respectively). From Table II and Fig. 4, HPMC gels show significant higher drug permeability and SSTF than cholesterol free Sp 40 and Sp 60 proniosomal systems (P < 0.05). Moreover, there were no significant differences in drug permeability between HPMC gels, proniosomes containing cholesterol of Sp 40 and Sp 60, and gels of Sp 20 and Sp 80 at 50% cholesterol (P > 0.05).
It was observed that HPMC gel systems showed lag times for drug permeation which were quite smaller than that for distilled water. A lag time of about 43.8, 39.29, and 43.31 min was required for flurbiprofen permeation from distilled water, HPMC gel, and alcoholic HPMC gel, respectively (Fig. 4). Interestingly, no time lag in the permeation of flurbiprofen from Proniosomes, however, they should be hydrated to form niosomes before drug release and permeation across rabbit skin. This might be due to water permeation from the receptor compartment to the skin, drug release from reconstituted vesicles and the permeation of the dissolved drug occurred very rapidly (33) or, due to the minimum sampling time of 1 h and the drug release was linear (R ≥ 0.9) and constantly slow (Table II) (8).
Release Versus Permeation Studies
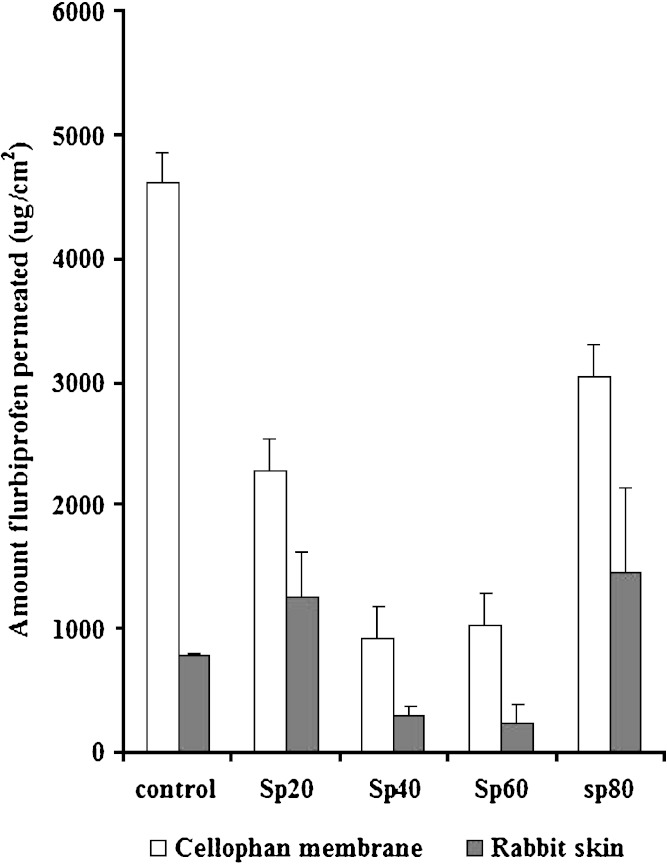
Figure 5 shows the amount flurbiprofen released across semipermeable cellophane membrane compared to that permeated across rabbit skin from cholesterol free proniosomal systems and the control after 8 h. The amounts of flurbiprofen released from cellophane membrane are significantly higher compared to the amounts permeated through the skin (P < 0.05) from the same formulation. This indicates the barrier properties of the skin for the drug (26). Moreover, all proniosomal systems of different spans gave much lower release rates than that of the control using cellophane membrane. On the other hand, permeation of flurbiprofen through rabbit skin is affected by the composition of proniosomes. Both cholesterol free Sp 20 and Sp 80 systems showed greater amounts of the drug permeated compared to the control (enhancement effect). However, cholesterol free Sp 40 and Sp 60 show lower permeation amounts of the drug (controlled release effects). The enhancement effects of Sp 20 and Sp 80 at lower cholesterol contents and the controlled release effects of both Sp 40 and Sp 60 proniosomes were explained before.
Fig. 5.
Amount drug released through cellophane membrane or permeated through rabbit skin from cholesterol free proniosomes and the control (flurbiprofen suspension) after 8 h. (n = 3)
CONCLUSION
In this investigation, proniosomal systems of different non-ionic surfactants which are available and cheap are easily prepared. The transition temperature of the used surfactant and cholesterol amount are the main factors affecting the type of proniosomal system, the in vitro release and permeation of flurbiprofen, and the mechanism by which the cholesterol can increase or decrease the drug flux. However, in vivo studies are required to prove the possibility of using proniosomes as vehicles for topical drug delivery.
References
- 1.Reddy D. N., Udupa N. Formulation and evaluation of oral and transdermal preparations of flurbiprofen and piroxicam incorporated with different carriers. Drug Dev. Ind. Pharm. 1993;19:843–852. doi: 10.3109/03639049309062986. [DOI] [Google Scholar]
- 2.Schreier H., Bouwstra J. Liposomes and niosomes as topical drug carriers dermal and transdermal drug delivery. J. Control. Rel. 1994;30:1–15. doi: 10.1016/0168-3659(94)90039-6. [DOI] [Google Scholar]
- 3.Vanhal D., Vanrensen A., Devringer T., Junginger H., Bouwstra J. Diffusion of estradiol from non ionic surfactant vesicles through human stratum corneum in vitro. STP Pharm. Sci. 1996;6:72–78. [Google Scholar]
- 4.Uchegbu I. F., Vyas S. P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 5.Varshosaz J., Pardakhty A., Seied M. H. B. Sorbitan monopalmitate-based proniosomes for transdermal delivery of chlorpheniramine maleate. Drug Deliv. 2005;12:75–82. doi: 10.1080/10717540490446044. [DOI] [PubMed] [Google Scholar]
- 6.Hofland H. E. J., Bouwstra J. A., Ponec M., Bodde H. E., Spies F., Coos Verhoef J., Junginger H. E. Interactions of non ionic surfactant vesicles with cultured keratinocytes and human skin in vitro: A survey of toxicological aspects and ultrastructural changes in stratum corneum. J. Control. Rel. 1991;16:155–168. doi: 10.1016/0168-3659(91)90039-G. [DOI] [Google Scholar]
- 7.Aggarwal D., Pal D., Mitra A. K., Kaur I. P. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int. J. Pharm. 2007;338:21–26. doi: 10.1016/j.ijpharm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Vora B., Khopade A. J., Jain N. K. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J. Control. Rel. 1998;54:149–165. doi: 10.1016/S0168-3659(97)00100-4. [DOI] [PubMed] [Google Scholar]
- 9.Anderson B. D., Conradi R. A. Predictive relationships in the water solubility of salts of a non steroidal anti-inflammatory drug. J. Pharm. Sci. 1985;74:815–820. doi: 10.1002/jps.2600740803. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa H., Sakai T., Saito H., Mori M., Tazoe R., Sugibayashi T., Nomura A. Anti-inflammatory and analgesic actions and skin irritation of flurbiprofen by dermal application. Lyakuhin Kenkyu. 1993;13:869–878. [Google Scholar]
- 11.Kyuki K., Tsurumi K., Fujimura H., Masumoto S., Hashimoto Y. Anti-inflammatory effect of flurbiprofen for dermal application (FP-A) Iyakuhin Kenkyu. 1984;15:293–298. [Google Scholar]
- 12.Masumoto S., Akiba T., Okumura M., Ichikawa K., Hashimoto Y. Anti-inflammatory effect of flurbiprofen by topical application. Iyakuhin Kenkyu. 1982;13:878–885. [Google Scholar]
- 13.A. H. Kibbe (ed), Handbook of Pharmaceutical Excepients, 3rd edn. American Pharmaceutical Association, Washington, D.C, 2000, pp. 511–514.
- 14.Sebastiani P., Nicoli S., Santi P. Effect of lactic acid and iontophoresis on drug permeation across rabbit ear skin. Int. J. Pharm. 2005;292:119–126. doi: 10.1016/j.ijpharm.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Larrucea E., Arellano A., Santoyo S., Ygartua P. Interaction of tenoxicam with cyclodextrins and its influence on the in vitro percutaneous penetration of the drug. Drug Dev. Ind. Pharm. 2001;27((3)):251–260. doi: 10.1081/DDC-100000243. [DOI] [PubMed] [Google Scholar]
- 16.Mura P., Faucci M.T., Bramanti G., Corti P. Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur. J. Pharm. Sci. 2000;9:365–372. doi: 10.1016/S0928-0987(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 17.Attwood D., Florence A. T. Surfactant systems, their chemistry, pharmacy and biology. London: Chapman and Hall; 1983.. [Google Scholar]
- 18.Sudaxshiina M., Benedicte Van Den B., Gregory G., Alexander T. F. Water in sorbitan monostearate organogels (water in oil gels) J. Pharm. Sci. 1999a;88((6)):615–619. doi: 10.1021/js980343j. [DOI] [PubMed] [Google Scholar]
- 19.Sudaxshiina M., Gregory G., Alexander T. F. Interaction of nonionic surfactant based organogel with aqueous media. Int. J. Pharm. 1999b;180:211–214. doi: 10.1016/S0378-5173(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 20.Martin A., Swarbrick J., Cammarata A. Physical Pharmacy. 3rd ed. Philadelphia: Lea and Febiger; 1993. pp. 496–501. [Google Scholar]
- 21.Godin B., Touitou E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliver. Rev. 2007;59:1152–1161. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka T., Sternberg B., Florence A. T. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and sorbitan triester (Span 85) Int. J. Pharm. 1994;105:1–6. doi: 10.1016/0378-5173(94)90228-3. [DOI] [Google Scholar]
- 23.Fang J. Y., Yu S. Y., Wu P. C., Huang Y. B., Tsai Y. H. In vitro skin permeation of estradiol from various proniosome formulations. Int. J. Pharm. 2001;215:91–99. doi: 10.1016/S0378-5173(00)00669-4. [DOI] [PubMed] [Google Scholar]
- 24.Valenta C., Wanka M., Heidlas J. Evaluation of novel soyalecithin formulations for dermal use containing ketoprofen as a model drug. J. Control. Rel. 2000;63:165–173. doi: 10.1016/S0168-3659(99)00199-6. [DOI] [PubMed] [Google Scholar]
- 25.Gupta P. N., Mishra V., Rawat A., Dubey P., Mahor S., Jain S., Chatterji D. P., Vyas S. P. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: A comparative study. Int. J. Pharm. 2005;293((1–2)):73–82. doi: 10.1016/j.ijpharm.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin–cholesterol–water interactions by differential scanning calorimetry and x-ray diffraction. Biochim. Biophys. Acta. 1968;150:333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- 27.Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transition in phospholipid vesicles: fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 1973;311:330–334. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 28.Alsarra I. A., Bosela A. A., Ahmed S. M., Mahrous G. M. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur. J. Pharm. and Biopharm. 2004;Xx:1–6. doi: 10.1016/j.ejpb.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim H. G. Release studies from lyotropic liquid crystal systems. J. Pharm. Sci. 1989;78((8)):683–687. doi: 10.1002/jps.2600780816. [DOI] [PubMed] [Google Scholar]
- 30.Ramírez-Campos M., Villafuerte-Robles L. Effect of formulation variables on verapamil hydrochloride release from hydrated HPMC matrices. Rev. Soc. Quím. Méx. 2004;48:326–331. [Google Scholar]
- 31.Parikh D. K., Ghosh T. K. Feasibility of transdermal delivery of fluoxetine. AAPS PharmSciTech. 2005;6((2)):144–149. doi: 10.1208/pt060222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megrab N. A., Williams A. C., Barry B. W. Oestradiol permeation across human skin, silastic and snake skin membranes: The effects of ethanol/water co-solvent systems. Int. J. Pharm. 1995;116:101–112. doi: 10.1016/0378-5173(94)00321-U. [DOI] [Google Scholar]
- 33.Hwang B. Y., Jung B. H., Chung S. J., Lee M. H., Shim C. K. In vitro skin permeation of nicotine from proliposomes. J. Control. Rel. 1997;49:177–184. doi: 10.1016/S0168-3659(97)00073-4. [DOI] [Google Scholar]