Abstract
A live attenuated H7N7 candidate vaccine virus was generated by reverse genetics using the modified hemagglutinin (HA) and neuraminidase (NA) genes of highly pathogenic (HP) A/Netherlands/219/03 (NL/03) (H7N7) wild-type (wt) virus and the six internal protein genes of the cold-adapted (ca) A/Ann Arbor/6/60 ca (AA ca) (H2N2) virus. The reassortant H7N7 NL/03 ca vaccine virus was temperature sensitive and attenuated in mice, ferrets, and African green monkeys (AGMs). Intranasal (i.n.) administration of a single dose of the H7N7 NL/03 ca vaccine virus fully protected mice from lethal challenge with homologous and heterologous H7 viruses from Eurasian and North American lineages. Two doses of the H7N7 NL/03 ca vaccine induced neutralizing antibodies in serum and provided complete protection from pulmonary replication of homologous and heterologous wild-type H7 challenge viruses in mice and ferrets. One dose of the H7N7 NL/03 ca vaccine elicited an antibody response in one of three AGMs that was completely protected from pulmonary replication of the homologous wild-type H7 challenge virus. The contribution of CD8+ and/or CD4+ T cells to the vaccine-induced protection of mice was evaluated by T-cell depletion; T lymphocytes were not essential for the vaccine-induced protection from lethal challenge with H7 wt viruses. Additionally, passively transferred neutralizing antibody induced by the H7N7 NL/03 ca virus protected mice from lethality following challenge with H7 wt viruses. The safety, immunogenicity, and efficacy of the H7N7 NL/03 ca vaccine virus in mice, ferrets, and AGMs support the evaluation of this vaccine virus in phase I clinical trials.
Highly pathogenic avian influenza (HPAI) is a disease of poultry that is caused by H5 or H7 avian influenza viruses and is associated with up to 100% mortality (2). Influenza A H7 subtype viruses from both Eurasian and North American lineages have resulted in more than 100 cases of human infection since 2002 in the Netherlands, Italy, Canada, the United Kingdom, and the United States. These cases include outbreaks of HPAI H7N7 virus in the Netherlands in 2003 that resulted in more than 80 cases of human infection and one fatality; HPAI H7N3 virus in British Columbia, Canada, in 2004 that resulted in two cases of conjunctivitis; a cluster of human infections of low-pathogenicity avian influenza (LPAI) H7N2 virus in the United Kingdom in 2007 that resulted in several cases of influenza-like illness and conjunctivitis; and a single case of respiratory infection in New York in 2003 (3-6, 17, 27).
Due to an unprecedented geographic spread of H5 subtype viruses since 2003 and the continued occurrence of sporadic cases of H5N1 infections in humans, much emphasis has been placed on the pandemic threat posed by H5 subtype viruses. However, H7 subtype viruses also have significant pandemic potential. Humans are immunologically naïve to the H7 avian influenza viruses (16), and LPAI H7 subtype viruses circulating in domestic poultry and wild birds in Eurasia and North America have the potential to evolve and acquire an HP phenotype either by accumulating mutations or by recombination at the hemagglutinin (HA) cleavage site resulting in a highly cleavable HA that is a virulence motif in poultry (30, 33, 34). Recent work also suggests that contemporary North American lineage H7 subtype viruses, isolated in 2002 to 2003, are partially adapted to recognize α2-6-linked sialic acids, which are the receptors preferred by human influenza viruses and are preferentially found in the human upper respiratory tract (7). Moreover, coinfection and genetic reassortment of RNA genomes between H7 avian influenza viruses and human influenza viruses, including the seasonal H1N1 and H3N2 and pandemic H1N1 viruses, could result in the generation of reassortant viruses with the capacity to efficiently transmit among people and result in a pandemic. Domesticated birds may serve as important intermediate hosts for the transmission of wild-bird influenza viruses to humans, as may pigs, as evidenced by human infections with swine-origin 2009 pandemic H1N1 influenza virus throughout the world.
Vaccination is the most effective method for the prevention of influenza. However, technical limitations result in delays in the rapid generation and availability of a strain-specific vaccine against an emerging pandemic virus. The emergence of antigenically distinct virus clades poses a substantial challenge for the design of vaccines against H5N1 viruses because of the possible need for clade-specific vaccines (1). Similar challenges are present for the generation of H7 subtype vaccine candidates, because antigenically distinct H7 subtype viruses, including North American lineage H7N2 and H7N3 and Eurasian lineage H7N7 and H7N3 viruses, have caused human disease. The successful control of H7 influenza virus in poultry has been achieved by stamping out and by vaccination of poultry (9). Vaccines for human use against both lineages of H7 influenza virus are under development, and candidate vaccines have been evaluated in preclinical and clinical studies (14, 23, 29, 42).
We have previously analyzed the antigenic relatedness among H7 viruses from Eurasian and North American lineages using postinfection mouse and ferret sera (22). Among 10 H7 viruses tested, A/Netherlands/219/03 (H7N7) virus induced the most broadly cross-neutralizing antibodies (Abs) (22). Based on the phylogenetic relationships and its ability to induce broadly cross-neutralizing antibodies in mice and ferrets, we selected the A/Netherlands/219/03 (NL/03) (H7N7) virus from the Eurasian lineage for vaccine development. We used reverse genetics to generate a live attenuated cold-adapted (ca) H7N7 candidate vaccine virus bearing a modified HA, a wild-type (wt) neuraminidase (NA) gene from the NL/03 wt virus, and the six internal protein gene segments from the cold-adapted (ca) influenza A virus vaccine donor strain, A/Ann Arbor/6/60 ca (AA ca) (H2N2). The immunogenicity and protective efficacy against challenge with HP and LP H7 viruses from the Eurasian and North American lineages of the reassortant H7N7 NL03/AA ca vaccine virus were evaluated in mice, ferrets, and African green monkeys (AGMs).
MATERIALS AND METHODS
Viruses.
The HPAI A/Netherlands/219/03 (H7N7) wt virus was the source of the HA and NA genes for the live attenuated ca reassortant vaccine virus H7N7 NL/03 ca. The six internal protein gene segments of the H7N7 NL/03 ca virus were derived from the AA ca donor virus. Influenza A/Ann Arbor/6/60 (H2N2) (AA) wt and AA ca viruses were provided by MedImmune. Additional viruses used for the evaluation of the efficacy of the vaccine include the HPAI virus A/chicken/British Columbia/CN-7/04 (ck/BC/04) (H7N3) wt, from the North American lineage and HP viruses A/Netherlands/219/03 (NL/03) (H7N7) wt and A/turkey/England/63 (tk/EG/63) (H7N3) and LP virus A/turkey/Italy/2379/99 (tk/Italy/99) (H7N1), from the Eurasian lineage. These viruses were kindly provided by Robert G. Webster, St. Jude Children's Research Hospital, Memphis, TN; David Swayne, Southeast Poultry Research Laboratory, U.S. Department of Agriculture (USDA), Athens, GA; Nancy Cox, Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA; and John Pasick, Canadian Food Inspection Agency, National Centre for Foreign Animal Disease, Winnipeg, Canada.
Virus stocks for the wt viruses were propagated in the allantoic cavity of 9- to 11-day-old embryonated specific-pathogen-free (SPF) hen's eggs at 37°C. The allantoic fluid from eggs inoculated with wt viruses was harvested 24 h postinoculation and tested for hemagglutinating activity. Eggs inoculated with ca reassortant viruses were incubated at 33°C and were harvested at 3 days postinoculation. Infectious allantoic fluids were pooled, divided into aliquots, and stored at −80°C until use. The 50% tissue culture infectious dose (TCID50) for each virus was determined by titration of serially diluted virus in Madin-Darby canine kidney (MDCK) cells and calculated by the method developed by Reed and Muench (32).
All experiments, including animal studies with HP H7 AI viruses and the reassortant virus, were conducted by using enhanced biosafety level 3 (BSL-3) containment procedures in laboratories approved for use by the U.S. Department of Agriculture and Centers for Disease Control and Prevention. Studies with mice were conducted at the National Institutes of Health (NIH), studies with ferrets and AGMs were conducted at Bioqual, Inc. (Rockville, MD), and studies with chickens were conducted at the Southeast Poultry Research Laboratory (SEPRL). Experiments with mice, ferrets, and AGMs were approved by the National Institutes of Health Animal Care and Use Committee. Experimental studies with chickens were approved by the USDA (SEPRL) Animal Care and Use Committee.
Plasmids and transfections.
cDNAs generated by reverse transcription (RT)-PCR from all six internal protein gene segments of the AA ca virus were cloned into plasmid pAD3000. This plasmid is a derivative of plasmid pHW2000 (18) that contains polymerase I (Pol I) and Pol II promoters for the expression of viral RNA (vRNA) and mRNA, respectively, from the full-length viral gene segment insert. Similarly, cDNAs derived by RT-PCR of the wt H7 HA gene, with a deletion and a point mutation of the sequence encoding 3 basic amino acids at the HA1-HA2 cleavage site, and the wt N7 NA gene from the H7N7 NL/03 virus were cloned into pAD3000. Sequences of all of the inserts were confirmed. For the H7N7 reassortant virus, the six plasmid DNAs encoding the internal protein genes of the AA ca virus were combined with the two plasmids encoding the modified H7 gene and wt N7 gene, and Vero cells were transfected with the eight-plasmid mixture by electroporation. Seed virus stocks were generated by limiting dilution in SPF eggs, followed by expansion in SPF eggs.
Phenotypic analysis of the reassortant viruses.
The temperature sensitivity (ts) phenotype of the parent and reassortant viruses was assessed by evaluating the cytopathic effect (CPE) in primary chick kidney (PCK) cells at 33°C and 39°C. Viruses that displayed a ≥100-fold reduction in titer at 39°C compared with that observed at the permissive temperature (33°C) were considered ts.
The ca phenotypes of the parent and reassortant viruses were determined by comparing their infectivities in PCK cells at 25°C and 33°C. Cold-adapted viruses can replicate efficiently at low temperatures; the ca phenotype is defined as a less-than-100-fold reduction of the virus titer at 25°C compared with that at 33°C. Cells incubated at 33°C or 39°C were examined for CPE at 6 days postinfection (dpi), and cells incubated at 25°C were examined for CPE at 10 days postinfection.
Plaque assay with and without trypsin.
Chicken embryo fibroblast (CEF) cells in six-well tissue culture plates were inoculated with 0.1 ml of virus serially diluted in Leibowitz (L-15) medium. The H7N7 NL/03 wt and ca viruses were adsorbed for 1 h, with shaking every 15 min. Wells were overlaid with 1.8% (wt/vol) Bacto agar (Difco) mixed 1:1 with 2× medium 199 containing antibiotics and amphotericin B (Fungizone) with or without 0.1 μg/ml porcine tosyl phenylalanyl chloromethyl ketone (TPCK) trypsin (Sigma). Plates were incubated at 33°C and 37°C for 4 to 5 days. Agarose overlays were carefully dislodged, and the cell monolayers were fixed with 100% methanol. Cell monolayers in each well were rinsed with 2 ml of Blotto (5% nonfat dry milk in 1× phosphate-buffered saline [PBS]) and incubated for 1 h at room temperature with 0.5 ml/well of a 1:1,000 dilution of the primary antibody in Blotto (chicken anti-AA ca antibody; MedImmune). Plates were washed three times with Blotto and incubated with 0.5 ml/well of a 1:2,000 dilution of rabbit anti-chicken IgY H&L horseradish peroxidase (HRP) (Pierce, Rockford, IL) secondary antibody in Blotto for 1 h at room temperature. Plates were washed three times with PBS, and plaques were stained by using 0.5 ml/well of 3-amino-9-ethylcarbazole (AEC) substrate solution (Dako, Carpinteria, CA) at 37°C for 30 to 45 min. Plates were washed once with water and dried at room temperature. The number of plaques in each well was counted, and titers were recorded as PFU per ml.
Pathogenicity and infectivity studies in chickens.
An intravenous (i.v.) pathogenicity test (38) was used to determine the pathogenicity of the H7N7 NL/03 ca virus for chickens. Eight 4-week-old White Leghorn chickens were inoculated i.v. with the H7N7 NL/03 ca virus at a dose of 0.2 ml of a 1:10 dilution of stock virus and were monitored for mortality up to 10 dpi. To determine infectivity via a simulated natural route of exposure, a separate group of 10 chickens was inoculated intranasally (i.n.) with 106 TCID50 of the H7N7 NL/03 ca virus. Oropharyngeal and cloacal swabs were collected for virus isolation at 3 dpi. Two birds from the i.n. inoculated group were euthanized at 3 dpi for virus isolation from kidney, heart, brain, and lungs. All the remaining chickens were euthanized and bled at 14 dpi, and sera were tested for evidence of seroconversion by an agar gel precipitin assay.
Pathogenicity and replication studies in mice.
To determine the 50% lethal dose (LD50) of the H7N7 wt and reassortant ca viruses, groups of 4- to 6-week-old female BALB/c mice were anesthetized and infected i.n. with serial 10-fold dilutions of the viruses in 50 μl. Mice were monitored daily for 14 dpi for mortality.
To assess the ability of the viruses to replicate in different organs, groups of 12 female BALB/c mice were inoculated i.n. with 106 TCID50 of the H7N7 NL/03 wt, H7N7 NL/03 ca, AA wt, or AA ca virus. At 2, 3, and 4 dpi, four mice from each group were euthanized, and lungs, nasal turbinates, spleen, and brains were harvested, weighed, and homogenized in L-15 medium containing antibiotics to make a 10% (wt/vol) tissue homogenate. Titers of tissue homogenates clarified by low-speed centrifugation in 24- and 96-well culture plates containing MDCK cells were determined, and titers are expressed as log10 TCID50/g of tissue.
Replication in ferrets.
The abilities of H7N7 ca and wt viruses to replicate in ferrets were compared. Groups of three 8- to 10-week-old ferrets (Simonsen Laboratories, CA) were prebled, tested by a hemagglutination inhibition assay, and found to be seronegative for antibodies to seasonal H3N2 and H1N1 influenza viruses. Each ferret was inoculated i.n. with 107 TCID50 of the H7N7 NL/03 ca vaccine or the H7N7 NL/03 wt virus in a volume of 0.2 ml (0.1 ml per nostril). At 3 dpi, ferrets were euthanized, and nasal turbinates, lungs, olfactory bulbs, and brain tissue were harvested. The nasal turbinates, olfactory bulbs, and brain tissue were titrated on MDCK monolayers as described above, and titers are expressed as log10 TCID50/g of tissue. The lung tissues were homogenized, serial 10-fold dilutions were prepared, and 0.1 ml was inoculated into four 9- to 11-day-old embryonated SPF hen's eggs. Eggs were incubated at 33°C for 72 h for the ca vaccine virus or 37°C for 24 h for wt virus. Allantoic fluid from each egg was subjected to a hemagglutination assay using 0.5% turkey red blood cells. Virus titers were determined as the 50% egg infectious dose (EID50) per gram of tissue by using the method of Reed and Muench (32).
Replication in African green monkeys.
Adult AGMs (Chlorocebus aethiops), male and female, were used. Four AGMs were inoculated with 2 × 106 TCID50 of virus (1.0 ml of 1 × 106 TCID50 i.n. plus 1.0 ml of 1 × 106 TCID50 intratracheally [i.t.]); biological samples, including nasal and pharyngeal swabs, and nasal wash samples were collected daily; and tracheal lavage fluid was collected every other day. Tissue samples, including lungs, trachea, and nasal turbinates, were collected at necropsy 2 and 4 days after viral inoculation, and the virus titers were measured on MDCK cells. For sampling of lung tissue, small portions of distal and proximal sites of cranial and caudal lobes from both sides (right and left) of the lungs were harvested. The lung titer for each animal is expressed as a mean titer ± the standard error (SE) for eight samples.
Evaluation of immunogenicity of the vaccine candidate in mice and ferrets.
Groups of four 4- to 6-week-old female BALB/c mice were immunized i.n. with 1 or 2 doses (28 days apart) of 50 μl containing 106 TCID50 of the H7N7 NL/03 ca vaccine virus or L-15 medium (mock immunized), and sera were collected from these mice on 0, 28, and 56 dpi. Similarly, groups of three 8- to 10-week-old ferrets that were seronegative for antibodies to circulating H3N2 and H1N1 human influenza viruses were immunized i.n. with 1 or 2 doses (28 days apart) of 0.2 ml (0.1 ml/nostril) containing 107 TCID50 of the H7N7 NL/03 ca virus or L-15 medium (mock immunized). Sera were collected at 0, 28, and 56 dpi. Neutralizing antibody titers in the postimmunization sera against the homologous and heterologous wt H7 viruses were determined in a microneutralization (MN) assay as described previously (23). The neutralizing antibody titer was defined as the reciprocal of the highest dilution of serum that completely neutralized the infectivity of 100 TCID50 of the virus as determined by the absence of CPE at day 4.
Evaluation of the efficacy of the vaccine candidate in mice. (i) Protection from lethality.
Groups of 4- to 6-week-old female BALB/c mice were immunized i.n. with 1 dose of 106 TCID50 of H7N7 NL/03 ca or L-15 medium (mock immunized). Groups of five mice were challenged at 4 weeks postimmunization with 50 μl containing 30 times the LD50 of the homologous H7N7 NL/03 wt virus, a heterologous ck/BC/04 (H7N3) wt virus from the North American lineage, and a heterologous tk/EG/63 (H7N3) wt virus from the Eurasian lineage. Mice were monitored daily for 14 days postchallenge.
(ii) Protection from replication of the challenge virus in mice.
The level of pulmonary replication and extrapulmonary spread of the challenge viruses was evaluated in mice. Groups of four mice that received 1 or 2 doses of 106 TCID50 of the H7N7 NL/03 ca vaccine candidate or L-15 medium (mock immunized) were challenged 4 weeks later with 105 TCID50 of the homologous H7N7 NL/03 wt virus or heterologous ck/BC/04 (H7N3) wt, tk/EG/63 (H7N3) wt, or tk/Italy/99 (H7N1) wt virus from the North American and Eurasian lineages. At 2 and 4 dpi, nasal turbinates, lungs, spleen, and brains were harvested and homogenized, and the titers were determined on MDCK cells. Log-transformed viral titers were compared by using the Mann-Whitney U test.
Evaluation of the efficacy of the vaccine candidate in ferrets.
The level of pulmonary replication and extrapulmonary spread of the challenge viruses in ferrets was evaluated. Groups of three 8- to 10-week-old ferrets that were seronegative for antibodies to circulating H3N2 and H1N1 human influenza viruses were immunized i.n. with 1 or 2 doses of 107 TCID50 of H7N7 NL/03 ca or L-15 medium (mock immunized) in a volume of 0.2 ml/ferret (0.1 ml/nostril). Ferrets were challenged 4 weeks after dose 1 or 2 with 106 TCID50 of the homologous H7N7 NL/03 wt virus and heterologous ck/BC/04 (H7N3) wt or tk/EG/63 (H7N3) wt virus from the North American and Eurasian lineages. Ferrets were euthanized 3 days postchallenge, and nasal turbinates, lungs (right and left lower lobes), and brains were harvested and homogenized. Tissue homogenates of nasal turbinates and brain tissues were titrated in MDCK cells, and virus titers were determined and expressed as log10 TCID50/g of tissue. Because embryonated eggs are more sensitive for detecting low concentrations of virus, lung homogenates from the ferrets were titrated in embryonated eggs, and virus titers are expressed as EID50/g of tissue. Log-transformed viral titers were compared by using the Mann-Whitney U test.
Evaluation of the immunogenicity and efficacy of the vaccine in African green monkeys.
AGMs were inoculated with the H7N7 NL/03 ca virus as indicated above. Sixty days after vaccination, AGMs were challenged with 2 × 106 TCID50 H7N7 NL/03 wt virus i.n. and i.t. Tissues were harvested at 2 days after challenge infection for virus titration as indicated above. Immune responses were assessed by measuring hemagglutination-inhibiting (HAI) and neutralizing antibodies in serum. HAI antibody titers were determined by standard methods, using 4 HA units of virus in V-bottom 96-well microtiter plates with 0.5% turkey erythrocytes. Neutralizing antibody titers were also evaluated with the MN assay, as described above.
Evaluation of the role of CD4+ and CD8+ T cells in vaccine-induced protection in mice.
Following 1 or 2 doses of the H7N7 NL/03 ca vaccine, CD4+ and/or CD8+ T cells were depleted in mice as described previously (12, 15) by the intraperitoneal (i.p.) injection of 1 mg/ml of anti-CD4 Ab (clone GK 1.5) and/or 1 mg/ml of anti-CD8 Ab (clone 2.43) 3 days before and after lethal challenge with H7 wt viruses. An irrelevant Ab (SFR-DR5) was used as a negative control. Mice were observed for signs of illness and weighed daily for 14 days.
Evaluation of the role of antibody in vaccine-induced protection of mice.
Postimmunization serum was generated in 50 mice that received 1 or 2 doses of the H7N7 NL/03 ca vaccine virus (106 TCID50) i.n. on days 0 and 28. On day 56, all the vaccinated mice were exsanguinated, sera collected from these mice were pooled, and a pool of nonimmune serum from uninfected mice was used as a control. In the passive-transfer experiment, groups of five naïve mice received 1 ml of a 1:2 or 1:10 dilution of sera collected after 1 or 2 doses of the H7N7 NL/03 ca vaccine by i.p. injection a day before challenge with 105 TCID50 of homologous H7N7 NL/03 wt or heterologous H7N3 ck/BC/04 wt, H7N3 tk/EG/63 wt, or H7N1 tk/Italy/99 wt virus. Mice were monitored daily for 14 days.
RESULTS
Generation of H7N7 NL/03 ca virus.
A reassortant virus containing a modified H7 HA gene, an N7 NA gene derived from the influenza A/Netherlands/219/03 (NL/03) (H7N7) wt virus, and the remaining gene segments from the AA ca virus was generated by plasmid-based reverse genetics as previously described (37). The HA gene was modified by the removal of the sequence encoding 3 basic amino acids at the HA cleavage site (Table 1) to generate a sequence that is present in avian influenza viruses that are not highly pathogenic for poultry. The reassortant virus was isolated and biologically cloned by limiting dilution in eggs. The nucleotide sequence of each gene segment of the NL/03 ca virus was confirmed to be identical to the sequence of the corresponding gene in the parent viruses. The five loci in the AA ca virus that specify temperature sensitivity (ts) and attenuation (att) phenotypes were present in the reassortant H7N7 NL/03 ca virus (20, 21).
TABLE 1.
The H7N7 NL/03 ca virus without the multibasic amino acid motif in the HA cleavage site requires trypsin for plaque formation in CEFs
| Virus | Amino acid sequence near the HA cleavage siteb | Titer in CEF cells (log10 PFU/ml) |
|
|---|---|---|---|
| With trypsin | Without trypsin | ||
| H7N7 NL/03 wt | PEIPKRRRR↓GLF | 8.6 | 8.6 |
| H7N7 NL/03 ca | PEIPK--GR↓GLF | 7.4 | <1.0a |
Lower limit of detection.
↓ indicates the site of cleavage of the HA into the HA1 and HA2 domains.
In vitro phenotypes: trypsin dependence and ts.
The HA gene in the H7N7 NL/03 ca virus differed from the HA gene in the corresponding NL/03 wt parent virus in the absence of the multibasic amino acid motif at the cleavage site. The presence of the multibasic amino acid sequence in the HA gene of an H7 or H5 subtype influenza A virus confers the ability to form plaques efficiently in CEF cells in the absence of trypsin (8, 31, 41). Accordingly, the H7N7 NL/03 wt virus that contains 5 basic amino acids at the HA cleavage site formed plaques in CEF cells equally efficiently in the presence or absence of trypsin (Table 1). However, the H7N7 NL/03 ca virus failed to form plaques in CEF cells in the absence of trypsin, consistent with the absence of the multibasic cleavage site motif in the modified HA protein (Table 1).
Previous studies with H1N1 and H3N2 reassortant ca viruses have shown that the five loci in the internal protein gene segments from the influenza AA ca virus, PB11195 (K391E), PB11766 (E581G), PB12005 (A661T), PB2821 (N265S), and NP146 (D34G), specify ts and att phenotypes (20, 21), and these phenotypes were seen in H9N2, H5N1, and H7N3 reassortant ca viruses (10, 23, 37). Consistent with previous findings, the H7N7 NL/03 ca and the parent AA ca viruses replicated equally well at 25°C and 33°C; thus, the NL/03 ca virus demonstrated the ca phenotype (Table 2). The H7N7 NL/03 ca and the parent AA ca viruses were restricted in replication at 39°C (ts); in contrast, the H7N7 NL/03 wt virus replicated equally well at 33°C and 39°C (Table 2). Thus, the H7N7 NL/03 ca virus possesses the ts phenotype. We have previously observed that the H5N1 wt virus was able to replicate efficiently at 25°C, indicating the variability of the biology of influenza viruses in nature (37), and in this experiment, the AA wt virus also possesses the ca phenotype (Table 2).
TABLE 2.
The H7N7 NL/03 ca reassortant virus possesses ca and ts phenotypes
| Virus | Mean titer ± SE (log10 TCID50/ml) |
Presence of ca phenotypea | Presence of ts phenotypeb | ||
|---|---|---|---|---|---|
| 25°C | 33°C | 39°C | |||
| AA wt | 8.6 ± 0.12 | 9.4 ± 0.06 | 9.1 ± 0.06 | + | − |
| AA ca | 8.3 ± 0.18 | 9.3 ± 0.10 | 5.3 ± 0.08 | + | + |
| H7N7 NL/03 wt | 5.75 ± 0.10 | 9.3 ± 0.21 | 9.4 ± 0.17 | − | − |
| H7N7 NL/03 ca | 6.43 ± 0.23 | 7.6 ± 0.08 | 4.5 ± 0.07 | + | + |
The ca phenotype is a ≤100-fold difference between the mean titers of the virus at 33°C and 25°C.
The ts phenotype is a ≥100-fold difference between the mean titers of the virus at 33°C and 39°C.
Attenuation in vivo in chickens, mice, ferrets, and African green monkeys. (i) Level of attenuation in chickens following i.v. and i.n. administration.
Clinical signs of illness were not observed in chickens upon i.n. and i.v. inoculation with the H7N7 NL/03 ca virus (Table 3). Consistent with the absence of the multibasic cleavage site motif in the HA protein, the H7N7 NL/03 ca virus was not lethal for chickens in an intravenous pathotyping test (Table 3), and virus was not isolated from oropharyngeal or cloacal swabs that were collected on day 3 following i.n. inoculation or from the kidney, heart, brain, and lungs of birds from the i.n. inoculated group that were euthanized at 3 dpi (data not shown). The H7N7 NL/03 ca virus also failed to elicit an antibody response following i.n. inoculation (Table 3). The poor replication of the H7N7 NL/03 ca virus in chickens is likely the result of an inability of this ts virus to replicate at the high body temperature of chickens (40°C to 41°C) or the consequence of six internal protein genes from a human influenza virus that fails to replicate efficiently in poultry and suggests that the use of the H7N7 NL/03 ca virus would not pose a threat to the poultry industry.
TABLE 3.
Assessment of pathogenicity and infectivity of the H7N7 NL/03 ca virus in chickens
| Virus | Route of administration | Total no. of chickens | Morbidity (no. of chickens) | Mortality (no. of survivors) | No. of chickens with antibody detected |
|---|---|---|---|---|---|
| H7N7 NL/03 ca | Intranasala | 8 | 0 | 0 | 0 |
| H7N7 NL/03 ca | Intravenousb | 8 | 0 | 0 | 2 |
106 EID50 of virus administered intranasally in a volume of 0.1 ml.
Groups of eight 4-week-old SPF chickens were inoculated i.v. with a 1:10 dilution of allantoic fluid.
(ii) Lethality in mice.
The H7N7 NL/03 wt and ca viruses were tested for lethality in mice. When administered intranasally, the H7N7 NL/03 wt virus was highly lethal for mice (LD50 = 102 TCID50), while the H7N7 NL/03 ca virus was not lethal for mice even at the highest dose tested (LD50 ≥ 106 TCID50).
(iii) Replication of the H7N7 NL/03 ca virus in mice.
The level of replication of the H7N7 NL/03 ca virus in mice was determined at 2, 3, and 4 dpi, and the differences were compared with those seen between the AA wt and ca viruses. The level of replication of the H7N7 NL/03 ca virus was significantly lower than that of the H7N7 wt virus in the upper (Fig. 1A) and lower (Fig. 1B) respiratory tracts of mice, and the differences in titer exceeded the difference seen between the titers achieved in mice infected with the AA wt and ca viruses. Although significant levels of NL/03 wt viruses were detected in the brain and spleen (103.2 TCID50/g and 104.5 TCID50/g, respectively), the H7N7 NL/03 ca virus was not detected in the brain and spleen (data not shown). Thus, the replication of the H7N7 NL/03 ca virus was highly restricted in mice compared to the H7N7 NL/03 wt virus.
FIG. 1.
Replication of the H7N7 NL/03 ca virus in mice. Shown are levels of replication of wt and ca viruses in mice following i.n. inoculation with 106 TCID50/mouse of the H7N7 NL/03 wt (•), H7N7 NL/03 ca (▪), AA wt (▴), and AA ca (⧫) viruses. Virus titers in the nasal turbinates (A) and lungs (B) of four mice per group sacrificed on 2, 3, or 4 dpi, respectively, are expressed as log10 TCID50/g of tissue. The solid line represents the mean for the group. The lower limit of detection is indicated by the dashed horizontal line. An asterisk indicates a statistically significant (P < 0.05) reduction in virus titers compared to the corresponding wt virus.
(iv) Replication of the H7N7 NL/03 ca virus in ferrets.
The level of replication of the H7N7 NL/03 ca virus was determined in ferrets at 3 dpi, and the difference was compared with that seen between the AA wt and ca viruses. While the H7N7 wt, AA wt, and AA ca viruses replicated to high titers in the upper respiratory tract (106.7 TCID50/g, 104.9 TCID50/g, and 105.5 TCID50/g, respectively), the H7N7 NL/03 ca virus was attenuated and replicated only to a moderate level, between 102 and 103 TCID50/g. The replication of the H7N7 NL/03 ca virus and AA ca virus was not detected in the lower respiratory tract of ferrets (Fig. 2), while the H7N7 NL/03 wt virus replicated to titers between 103 and 104 EID50/g in the lungs (Fig. 2). The H7N7 NL/03 ca, AA wt, and AA ca viruses were not detected in the brain and the olfactory bulb, but the H7N7 NL/03 wt virus was detected at moderate and high levels at these sites (104.5 TCID50/g and 106.5 TCID50/g, respectively) (Fig. 2). Thus, the H7N7 NL/03 ca virus was highly restricted in replication in the respiratory tract of ferrets compared to the corresponding wt virus, consistent with the observations of mice, and the virus demonstrated the att phenotype predicted by the genetic modifications engineered into the HA gene segment and the mutations in the internal gene segments that were derived from the AA ca virus.
FIG. 2.
Replication of the H7N7 NL/03 ca virus in ferrets. Shown are levels of replication of wt and ca viruses in ferrets following i.n. inoculation of 107 TCID50/ferret of the H7N7 NL/03 wt (•), H7N7 NL/03 ca (▪), AA wt (▴), and AA ca (⧫) viruses on 3 dpi. Virus titers in the indicated organs are expressed as log10 TCID50/g or log10 EID50/g of tissue. The solid line represents the mean for the group. The lower limit of detection is indicated by the dashed horizontal line. An asterisk indicates a statistically significant (P < 0.05) reduction in virus titers compared to the corresponding wt virus.
(v) Replication of the H7N7 NL/03 ca virus in AGMs.
Groups of four AGMs were inoculated with the H7N7 NL/03 wt and H7N7 NL/03 ca viruses, and two AGMs were euthanized at 2 dpi and 4 dpi, respectively, from each group. Respiratory tissues, including nasal turbinates, trachea, and lungs, were collected, and virus titers in the tissue homogenates were determined (Table 4). The H7N7 NL/03 wt virus replicated well in the upper and lower respiratory tracts. Virus was also detected in respiratory secretions, including nasal and pharyngeal swabs, nasal wash specimens, and tracheal lavage fluid. The virus titer in the tissue samples was slightly higher at 2 dpi than at 4 dpi, but the titers in secretions were variable (Table 4). In contrast, virus was detected in respiratory secretions but not in respiratory tissues of animals infected with the H7N7 NL/03 ca virus (Table 4).
TABLE 4.
Replication of H7N7 NL/03 wt and H7N7 NL/03 ca viruses in African green monkeys
| Virus | Day of necropsy | Animal | Virus titer in respiratory tissues (log10TCID50/g) |
Virus titer in respiratory secretions (log10TCID50/ml)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal turbinates | Trachea | Lungsa | Nasal/ pharyngeal swabs on day: |
Nasal wash specimens on day: |
Tracheal lavage fluid on day: |
||||||
| 2 | 4 | 2 | 4 | 2 | 4 | ||||||
| H7N7 NL/03 wt | 2 | 1 | 8.7 | 7.2 | 6.1 ± 1.77 | 3.2 | ND | 3.2 | ND | 3.2 | ND |
| 2 | 2 | 8.2 | 7.7 | 6.5 ± 1.21 | 3.2 | ND | 3.2 | ND | 3.2 | ND | |
| 4 | 3 | 6.7 | 6.4 | 5.1 ± 1.21 | 3.2 | 3.2 | 1.5 | <0.5c | ND | <0.5 | |
| 4 | 4 | 5.7 | 7 | 6.6 ± 0.41 | 3.1 | 6.7 | 5.2 | 1.5 | ND | <0.5 | |
| H7N7 NL/03 ca | 2 | 5 | <1.5d | <1.5 | <1.5 | 4.4 | ND | 3.2 | ND | 4.2 | ND |
| 2 | 6 | <1.5 | <1.5 | <1.5 | 3.9 | ND | 3.2 | ND | <0.5 | ND | |
| 4 | 7 | <1.5 | <1.5 | <1.5 | 5.2 | 3.4 | 3.2 | <0.5 | ND | 4.0 | |
| 4 | 8 | <1.5 | <1.5 | <1.5 | 4.2 | 4.2 | 2.4 | 4.0 | ND | <0.5 | |
Mean titer of eight lung samples of individual AGMs ± SE.
ND, not done.
Detection limit of virus in secretions.
Detection limit of virus in tissues.
Immunogenicity of the H7N7 NL/03 ca vaccine virus. (i) Immunogenicity in mice.
The immunogenicity of the H7N7 NL/03 ca virus in mice was evaluated following the i.n. administration of 1 or 2 doses of the vaccine virus. A single dose of the H7N7 NL/03 ca vaccine was poorly immunogenic in mice. It induced a low neutralizing antibody response against the homologous virus, with a geometric mean titer (GMT) of 14 and a range of 10 to 32 for individual mice on day 28 postimmunization (Table 5). However, the neutralizing antibody response increased significantly after a second dose of the H7N7 NL/03 ca virus, with a GMT of 191 and a range of 143 to 226 for individual mice on day 56 postimmunization (Table 5). The level of cross-neutralizing antibodies against heterologous ck/BC/04 (H7N3), tk/EG/63 (H7N3), and tk/Italy/99 (H7N1) wt viruses in the sera of mice was determined. Two doses of vaccine induced a robust cross-reactive antibody response against all four H7 viruses (Table 5).
TABLE 5.
Serum neutralizing antibodies elicited in mice and ferrets following 1 or 2 doses of H7N7 NL/03 ca vaccinea
| Species | No. of doses | No. of days postimmunization | Reciprocal GMT of serum neutralizing antibody against indicated wt virusb |
|||
|---|---|---|---|---|---|---|
| H7N7 NL/03 | H7N3 ck/BC/04 | H7N3 tk/EG/63 | H7N1 tk/Italy/99 | |||
| Mouse | 1 | 28 | 14 | 24 | 21 | 22 |
| 2 | 56 | 191 | 285 | 153 | 160 | |
| Ferret | 1 | 28 | 12 | 10 | 15 | ND |
| 2 | 56 | 41 | 54 | 80 | ND | |
Mice and ferrets received one or two intranasal doses of 106 TCID50 and 107 TCID50 of the immunizing virus, respectively.
Values represent reciprocal geometric mean antibody titers from four mice and three ferrets per group. Antibodies were not detected in preimmunization sera and in sera from mock-immunized mice and ferrets. An undetectable serum neutralizing antibody titer was assigned a value of 10. Homologous antibody titers are in boldface type. ND, not done.
(ii) Immunogenicity in ferrets.
The homologous H7N7 NL/03 wt virus, a heterologous ck/BC/04 (H7N3) wt virus from the North American lineage, and a heterologous tk/EG/63 (H7N3) wt virus from the Eurasian lineage were used for challenge studies in ferrets following 1 or 2 doses of the vaccine. Consistent with findings from the study of mice, after 1 dose of the H7N7 NL/03 ca virus, only a low level of neutralizing antibody was induced against the homologous wt virus (GMT of 12; range, 10 to 13); however, a higher GMT (41; range, 20 to 71) was detected following 2 doses of the vaccine (Table 5). The level of cross-neutralizing antibodies against the heterologous ck/BC/04 (H7N3) and tk/EG/63 (H7N3) wt viruses in the sera of ferrets was determined. One and two doses of the H7N7 NL/03 ca virus induced GMTs of 10 and 54, respectively, against the heterologous ck/BC/04 (H7N3) wt virus and GMTs of 15 and 80, respectively, against the heterologous tk/EG/63 (H7N3) wt virus (Table 5).
(iii) Immunogenicity in AGMs.
Three AGMs were immunized with 1 dose (2 × 106 TCID50/dose in 2 ml) of the H7N7 NL/03 ca virus. An additional AGM was inoculated with 2 ml of L-15 medium as a mock-vaccinated control. Blood samples were collected biweekly, and the sera were tested for HAI and neutralizing antibodies. All three AGMs developed neutralizing antibodies 2 weeks after vaccination with the H7N7 NL/03 ca virus, and the titer peaked at 6 or 8 weeks postvaccination (p.v.), with a range from 1:16 to 1:128 (Table 6). The AGM with the highest titer of neutralizing antibody had an HAI titer of 1:80 at day 56 p.v. HAI antibodies were not detected in the remaining AGMs (data not shown).
TABLE 6.
Immunogenicity and efficacy of the H7N7 NL/03 ca vaccine in African green monkeysa
| Vaccine | Animal | Prechallenge serum antibody titerb |
Titer of challenge virus in respiratory tissues (log10TCID50/g) |
Titer of challenge virus in respiratory secretions (log10TCID50/ml) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| HAI | NT | Nasal turbinates | Trachea | Lungs | Nasal/pharyngeal swabs | Nasal wash specimens | Tracheal lavage fluid | ||
| L-15 | 1 | <1:20c | <1:2d | 6 | 6.7 | 4.1 ± 2.1e | 1.0 | 5.7 | 5.4 |
| H7N7 NL/03 ca | 2 | 1:80 | 1:128 | <1.5f | <1.5 | <1.5 | <0.5g | <0.5 | <0.5 |
| H7N7 NL/03 ca | 3 | <1:20 | 1:64 | 3.4 | 4.7 | 2.8 ± 1.7 | <0.5 | <0.5 | <0.5 |
| H7N7 NL/03 ca | 4 | <1:20 | 1:16 | <1.5 | 4.3 | 4.7 ± 2.4 | <0.5 | <0.5 | <0.5 |
AGMs were immunized with a single dose of vaccine and were challenged with the H7N7 NL/03 wt virus 8 weeks later, and tissues were harvested 2 days following challenge.
Sera collected 8 weeks after immunization.
Lowest dilution of serum tested in an HAI assay.
Lowest dilution of serum tested in an MN assay.
Mean titer from eight lung samples ± SE.
Detection limit in tissues.
Detection limit in secretions.
Efficacy of the H7N7 NL/03 ca vaccine virus. (i) Protection from lethal challenge in mice.
Following immunization with 1 dose (106 TCID50/dose) of the H7N7 NL/03 ca virus, groups of five mice were challenged with 30 LD50 of the homologous H7N7 NL/03 wt virus, a heterologous ck/BC/04 (H7N3) wt virus from the North American lineage, and a heterologous tk/EG/63 (H7N3) wt virus from the Eurasian lineage. A single dose of the H7N7 NL/03 ca virus provided complete protection from lethal challenge with all three viruses, while mock-immunized mice succumbed to death on day 6 following challenge (data not shown).
(ii) Protection from replication of homologous and heterologous H7 challenge viruses in mice.
The efficacy of 1 or 2 doses of the H7N7 NL/03 ca virus (106 TCID50/dose) in preventing the replication of challenge viruses (105 TCID50/dose) was evaluated on days 2 and 4 following challenge, when all of the challenge viruses were predicted to be present at a high titer in the lungs of mock-immunized animals. The replication of challenge viruses in nasal turbinates, lung, spleen, and brain was evaluated in these mice. Both 1 and 2 doses of vaccination with H7N7 NL/03 ca virus conferred complete protection in the spleen and brain (data not shown). Two doses of the H7N7 NL/03 ca virus vaccine conferred near-complete protection from replication of the homologous NL/03 (H7N7) and heterologous ck/BC/04 (H7N3), tk/EG/63 (H7N3), and tk/Italy/99 (H7N1) wt viruses in the nasal turbinates (Fig. 3A) and lungs (Fig. 3B), while a significant reduction in titer in the upper respiratory tract (Fig. 3A) without complete protection from pulmonary virus replication was seen following a single dose of the vaccine (Fig. 3B). Complete protection from replication of H7 wt challenge viruses in the respiratory tract of mice following 2 doses of H7N7 NL/03 ca vaccine was consistent with a high level of neutralizing antibody response against these H7 wt viruses (Table 5).
FIG. 3.
Replication of H7 wt challenge viruses in the respiratory tract of mice immunized with the H7N7 NL/03 ca virus. Groups of four mice were immunized with 1 dose (upper panel) or 2 doses (lower panel) of 106 TCID50/mouse of the H7N7 NL/03 ca virus (○ and □) or were mock immunized (L-15) (• and ▪) and were challenged with 105 TCID50/mouse of each indicated virus. The H7N7 NL/03 wt virus is the homologous HPAI virus, and the H7N3 ck/BC/04 wt and tk/EG/63 wt viruses are heterologous HPAI viruses from North American and Eurasian lineages. The H7N1 tk/Italy/99 wt virus is a heterologous LPAI virus from the Eurasian lineage. Nasal turbinates (A) and lungs (B) were harvested 2 days (○ and •) and 4 days (□ and ▪) following challenge. Virus titers are expressed as log10 TCID50/g of tissue. The lower limit of detection is indicated by the dashed horizontal line. An asterisk indicates a statistically significant (P < 0.05) reduction in virus titers compared to the titers in the mock-immunized group.
(iii) Protection from replication of homologous and heterologous H7 challenge viruses in ferrets.
The homologous NL/03 (H7N7) virus and heterologous ck/BC/04 (H7N3) and tk/EG/63 (H7N3) wt viruses from the North American and Eurasian lineages, respectively, were also used for challenge studies in ferrets. Significant reductions (P < 0.05) in the levels of heterologous challenge virus replication were observed for the upper respiratory tract (Fig. 4A) when ferrets were challenged 4 weeks after 1 dose of the vaccine. Two doses of the vaccine resulted in a further reduction in the virus titers in the upper respiratory tract (Fig. 4A). A single dose of the H7N7 NL/03 ca vaccine provided complete protection from replication of the homologous and heterologous H7 wt challenge viruses in the lower respiratory tract (Fig. 4B). One dose of the vaccine also prevented the spread of the NL/03 (H7N7) wt virus to the brain and significantly reduced (P < 0.05) the titers of the challenge viruses in the olfactory bulb (data not shown).
FIG. 4.
Replication of H7 wt challenge viruses in the respiratory tract of ferrets immunized with the H7N7 NL/03 ca virus. Groups of ferrets were immunized with 1 dose (□) or 2 doses (⋄) of 107 TCID50/ferret of the H7N7 NL/03 ca virus or were mock immunized (L-15) (•), and four ferrets per group were challenged 4 weeks after doses 1 and 2 with 106 TCID50/ferret of each indicated virus. Nasal turbinates (A) and lungs (B) were harvested 3 days following challenge, homogenized, and titrated on MDCK cells and embryonated eggs, respectively. Virus titers are expressed as log10 TCID50/g or log10 EID50/g of tissue. The solid line represents the mean for the group. The lower limit of detection is indicated by the dashed horizontal line. An asterisk indicates a statistically significant (P < 0.05) reduction in virus titers compared to the titers in the mock-immunized group.
(iv) Protection from replication of homologous H7 challenge virus in AGMs.
The protective efficacy of the H7N7 NL/03 ca vaccine virus against H7N7 NL/03 wt virus challenge was evaluated in respiratory tissues and secretions harvested 2 days after challenge. Virus was detected in all tissues and secretions from the mock-vaccinated control animal. Virus was not detected in respiratory tissues or secretion samples of the AGM (animal 2) who developed high titers of serum HAI and neutralizing antibodies (Table 6), whereas virus was detected in respiratory tissues but not in secretions of the two AGMs (animals 3 and 4) who had lower serum neutralizing antibody titers and no detectable HAI antibodies (Table 6). Thus, the level of protection correlated well with serum neutralizing antibody titers elicited by the vaccine.
Evaluation of the immune mechanism underlying vaccine-induced protection in mice.
Because a single dose of the H7N7 NL/03 ca vaccine conferred protection from lethal challenge despite a modest neutralizing antibody response, we determined which effector arm of the immune system protected mice from lethal challenge with H7 wt viruses following 1 or 2 doses of the H7N7 NL/03 ca vaccine.
(i) Evaluation of the role of CD4+ and CD8+ T cells in vaccine efficacy.
In order to assess the role of T cells in vaccine-induced protection from homologous and heterologous H7 challenge viruses in mice, we depleted CD8+ and/or CD4+ T cells in mice that received 1 or 2 doses of vaccine by the i.p. injection of anti-CD8+ and/or CD4+ Abs 3 days before and after lethal challenge with H7 wt viruses. We used a protocol for T-cell depletion that was previously shown to deplete these T-cell subsets (12, 15). Despite the depletion of CD8+ and/or CD4+ T cells, all of the mice immunized with a single dose of the H7N7 NL/03 ca virus survived challenge with all three viruses, including the homologous NL/03 (H7N7) virus and the heterologous ck/BC/04 (H7N3) and tk/EG/63 (H7N3) wt viruses (data not shown). Therefore, we conclude that T lymphocytes are not essential for the protection of vaccinated mice from lethal challenge with H7 wt viruses.
(ii) Evaluation of the role of serum antibody in vaccine efficacy.
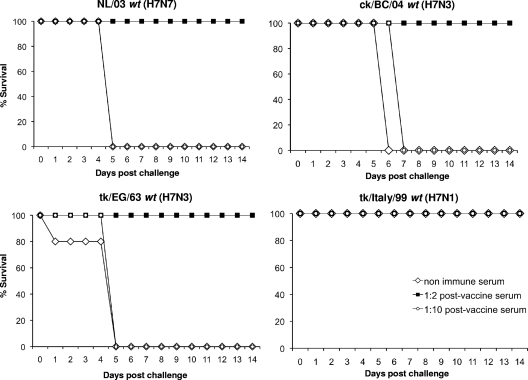
We generated postvaccination immune serum by administering 1 or 2 doses of the H7N7 NL/03 ca vaccine virus intranasally. We transferred a 1:2 or 1:10 dilution of postvaccination serum to naïve mice via i.p. injection to determine whether antibody alone could protect mice from challenge with the homologous H7N7 NL/03 wt or the heterologous H7N3 ck/BC/04 wt, H7N3 tk/EG/63 wt, or H7N1 tk/Italy/99 wt virus. Nonimmune serum from uninfected mice was administered to control groups. All of the mice that received a 1:2 dilution of the 1-dose postvaccination serum survived challenge with the homologous H7N7 NL/03 wt and heterologous H7N3 ck/BC/04 wt, H7N3 tk/EG/63 wt, and H7N1 tk/Italy/99 wt viruses (Fig. 5). Mice that received nonimmune serum died between 5 and 6 days postchallenge with the H7N7 NL/03 wt, H7N3 ck/BC/04 wt, or H7N3 tk/EG/63 wt virus (Fig. 5), as did mice that received a 1:10 dilution of the 1-dose postvaccination serum. Not surprisingly, all of the mice that received a 1:2 or 1:10 dilution of the 2-dose postvaccination serum survived challenge with the homologous H7N7 NL/03 wt or the heterologous H7N3 ck/BC/04 wt, H7N3 tk/EG/63 wt, or H7N1 tk/Italy/99 wt virus (data not shown). These data indicate that the passively transferred serum antibody induced by the H7N7 NL/03 ca vaccine mediates protection from lethality following challenge with H7 wt viruses in mice.
FIG. 5.
Survival of mice passively immunized with 1-dose postvaccination immune serum followed by challenge with H7 wt viruses. Groups of five naïve mice received a 1:2 (▪) or 1:10 (○) dilution of 1-dose postvaccination immune serum by i.p. injection a day before challenge with 105 TCID50 of the homologous H7N7 NL/03 wt or heterologous H7N3 ck/BC/04 wt, H7N3 tk/EG/63 wt, or H7N1 tk/Italy/99 wt virus. Nonimmune serum (⋄) was administered to the control group. Mice were monitored daily for 14 days, and percent survival over the course of the observation is shown.
DISCUSSION
The direct transmission of AI H7 subtype viruses from poultry to humans in the course of HPAI outbreaks in poultry in the Netherlands in 2003 and Canada in 2004 has resulted in mild to severe illness in people working closely with infected poultry (17, 26, 35, 40), including a case of acute fatal respiratory illness in the Netherlands during the HPAI H7N7 virus outbreak in chickens in 2003. As we learned from the H1N1 pandemic of 2009, it is difficult to predict which influenza virus will cross the host species barrier and cause a pandemic. Therefore, it is important to have a library of candidate vaccines for different subtypes that have been evaluated in animal models and in humans. In order to develop vaccines against H7 subtype viruses, we have previously evaluated 10 H7 viruses for their ability to induce high titers of a neutralizing antibody that was cross-reactive within the H7 subtype (22). Among these 10 viruses, the A/Netherlands/219/03 (H7N7) virus from the Eurasian lineage induced the most broadly cross-reactive neutralizing antibodies against both Eurasian and North American lineage viruses. We also selected A/ck/BC/CN-6/04 (H7N3) and generated a live attenuated H7N3 BC 04 ca vaccine virus that was evaluated in preclinical trials (23) and phase I clinical trials (39).
In this study, we generated a live attenuated H7N7 NL/03 ca vaccine virus by plasmid-based reverse genetics using the HA and NA genes of the H7N7 NL/03 wt virus and six internal protein genes of a live attenuated influenza A virus vaccine donor strain, the AA ca virus. Three basic amino acid residues at the cleavage site in the HA gene of the H7N7 NL/03 wt virus were genetically modified to remove the multibasic virulence motif, as described previously for H5N1 ca vaccines (37). Consistent with the absence of the multibasic amino acid motif at the cleavage site in HA, the H7N7 NL/03 ca vaccine virus failed to form plaques in CEF cells in the absence of exogenous trypsin and was not lethal for chickens. The H7N7 NL/03 ca vaccine virus displayed ca, ts, and att phenotypes that are specified by the internal protein genes of the AA ca virus (20, 21). The safety of the H7N7 NL/03 ca vaccine virus was established in mice, ferrets, and AGMs. The reassortant H7N7 NL/03 ca virus was not lethal for mice. Unlike the wt parent virus, the replication of the H7N7 NL/03 ca vaccine virus was highly restricted in the respiratory tract of mice, and extrapulmonary spread was not observed. The attenuation phenotype was also seen in the ferret and AGM models. In ferrets, the H7N7 NL/03 wt virus replicated to high titers in the upper and lower respiratory tracts, while the H7N7 NL/03 ca virus was attenuated in the nasal turbinates and was undetectable in the lungs, and there was no spread to the brain and spleen. These characteristics of the H7N7 NL/03 ca vaccine virus in ferrets fit the attenuation criteria for the AA ca-based live attenuated influenza virus vaccines that are licensed for human use. In AGMs, the H7N7 NL/03 wt virus replicated to high titers in the nasal turbinates, trachea, and lungs, while the H7N7 NL/03 ca virus was undetectable in all three of these tissues. However, the H7N7 NL/03 ca virus was found at low to moderate titers in respiratory secretions, indicating that the major site of replication for the H7N7 NL/03 ca virus in AGMs may be in the pharynx between the trachea and nasal cavity, and this site should be sampled in future studies. Based on our previous investigation of factors that contributed to the attenuation of an H5N1 ca vaccine (36), we infer that the attenuation of the H7N7 NL/03 ca vaccine virus in mice, ferrets, and AGMs can likely be attributed to the acquisition of the six internal protein gene segments from the AA ca virus and the removal of the multibasic cleavage site in the HA. Live attenuated ca H9N2, H5N1, and H7N3 avian influenza A subtype virus vaccines bearing the six internal protein genes of the AA ca virus have demonstrated similar levels of attenuation in mice and ferrets (10, 23, 37). Restricted replication in the respiratory tract and the lack of systemic spread in mice and ferrets suggest that the H7N7 NL/03 ca vaccine virus is attenuated and therefore safe for use in humans.
Two doses of the H7N7 NL/03 ca virus induced neutralizing antibodies with broad cross-reactivity in mice and ferrets, but a single dose of the H7N7 NL/03 ca vaccine virus was poorly immunogenic in mice and ferrets. A different phenomenon was observed in the case of the H7N3 ca vaccine, generated against an H7N3 isolate from Canada, where a single dose of the H7N3 BC 04 ca vaccine virus induced neutralizing antibodies in mice and ferrets (23). This difference in the immunogenicities of the two H7 ca candidate vaccines may be related to the level of replication of the vaccine viruses: the replication of the H7N3 BC 04 ca virus (104.5 TCID50/g and 104.9 TCID50/g in the nasal turbinates of mice and ferrets, respectively) was higher than that of the H7N7 NL/03 ca virus.
The efficacy of the H7N7 NL/03 ca vaccine in mice and ferrets was demonstrated by using challenge viruses that were selected based on our evaluation of several H7 viruses (22; our unpublished data). We have previously demonstrated that a single dose of the H5N1 ca or H7N3 ca vaccine provided complete protection from lethality following challenge with homologous and antigenically distinct heterologous H5N1 or H7N3 wt viruses, respectively (23, 37). In the case of the H7N7 ca vaccine as well, a single dose of the vaccine provided complete protection from lethal challenge with wt H7 viruses in mice. Because this protection was observed in the absence of a robust neutralizing antibody response, we were interested in elucidating the immunological basis for protection from lethal challenge. We found that the presence of T cells at the time of challenge was not essential for protection, while the passive transfer of serum from mice immunized with the H7N7 NL/03 ca virus to naïve mice protected the recipient mice from lethal challenge with H7 wt viruses, demonstrating the importance of antibodies in protection.
Two doses of the vaccine completely protected mice from pulmonary replication of homologous and heterologous viruses from the North American and Eurasian lineages and prevented the dissemination of the challenge viruses to the brain and spleen. This finding is consistent with the robust cross-neutralizing antibody response against these H7 wt viruses after 2 doses of the vaccine. In AGMs, the level of serum neutralizing antibody correlated well with the level of protection, supporting the role of humoral immunity in the protection observed for mice and ferrets. The data from preclinical studies are very valuable for two reasons, first because they provide data that can be used to correlate with immunogenicity data from clinical trials and second because they can provide scientific support for anticipated efficacy in humans, because challenge studies to assess the efficacy of pandemic influenza virus vaccines, such as H7N7 viruses, cannot be conducted in humans. We have previously evaluated the efficacy of H9N2 ca, H5N1 ca, and H7N3 ca vaccines in mice and ferrets. Although a single dose of the H9N2 ca and H7N3 ca vaccines, respectively, protected mice and ferrets from pulmonary replication of the homologous and antigenically heterologous wt challenge viruses (11, 23), 2 doses of H5N1 ca vaccines were needed to provide complete protection from pulmonary replication of homologous and antigenically distinct heterologous H5N1 wt viruses in mice and in ferrets (37). Taken together, we see a correlation between neutralizing antibody titers in the serum and protection from pulmonary replication of challenge viruses.
Inactivated vaccines against both lineages of H7 viruses on an A/PR/8/34 (PR/8) (H1N1) backbone have been evaluated in preclinical trials (14, 19, 29). Formalin-inactivated H7N2/PR8 and H7N7/PR8 whole-virus vaccines with and without aluminum hydroxide adjuvant elicited broadly cross-reactive antibodies against divergent H7 viruses and protected mice against challenge with heterologous H7 wt viruses of both Eurasian and North American lineages (14, 19, 29). Thus, H7N2, H7N7, H7N1, and H7N3 vaccines have been variably immunogenic yet generally protect experimental animals from challenge with homologous and heterologous H7 viruses. A cell-grown H7N1/PR8 vaccine virus containing the HA and NA genes from HPAI A/ck/Italy/99 (H7N1) virus has been evaluated with humans (13, 42). This vaccine induced low levels of HAI and neutralizing antibodies, although the addition of an aluminum hydroxide adjuvant enhanced the antibody responses. A live attenuated H7N3 ca vaccine generated in our laboratory was evaluated with healthy adults, and although the vaccine virus was highly restricted in replication, it was highly immunogenic (39). We selected the NL/03 virus as an additional vaccine target because this virus elicited the most broadly cross-reactive antibody response against a range of antigenically distinct H7 viruses (23). Based on the promising preclinical data, careful clinical evaluation of the H7N7 NL/03 ca vaccine is warranted. The risk of reassortment of a live attenuated pandemic influenza vaccine virus with a circulating human influenza virus, resulting in a novel subtype of influenza virus that could spread in the human population, is a significant concern. However, we believe that it is important to generate and carefully evaluate candidate live attenuated avian influenza virus vaccines in clinical trials with humans because of their potential advantages over other vaccine approaches (28, 37), and the risk of reassortment of the live attenuated vaccine virus with human influenza viruses during clinical trials can be minimized by conducting vaccine studies in isolation units when human influenza viruses are not circulating in the community (24, 25, 39). A live attenuated H7N7 vaccine will be administered to the public only if an influenza pandemic caused by a virus of the H7N7 subtype were imminent, and it will be used only upon the recommendation of public health authorities (28, 37).
Acknowledgments
We thank Jadon Jackson and the staff of the Comparative Medicine Branch, NIAID, for excellent technical support for animal studies performed at the NIH; Joan Beck for assistance in performing studies with chickens at SEPRL; and Nick Nguyen and staff of the Animal Care Facility of MedImmune for their assistance with ferret studies. We thank Zhaoti Wang, Helen Zhou, Winnie Chan, Brandon Liang, and Chin-Fen Yang at MedImmune for their excellent technical assistance. We thank Brad Finneyfrock and Anthony Cook for the ferret and AGM studies conducted at Bioqual. We are grateful to Robert G. Webster, Nancy Cox, and John Pasick for providing the viruses used in this study.
This research was performed as part of a Cooperative Research and Development Agreement (CRADA) between the Laboratory of Infectious Diseases, NIAID, and MedImmune and was supported in part by the Intramural Research Program of the NIAID, NIH.
Footnotes
Published ahead of print on 1 September 2010.
REFERENCES
- 1.Abdel-Ghafar, A. N., T. Chotpitayasunondh, Z. Gao, F. G. Hayden, D. H. Nguyen, M. D. de Jong, A. Naghdaliyev, J. S. Peiris, N. Shindo, S. Soeroso, and T. M. Uyeki. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261-273. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. J. 2000. A review of avian influenza in different bird species. Vet. Microbiol. 74:3-13. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2004. Update: influenza activity—United States and worldwide, 2003-04 season, and composition of the 2004-05 influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 53:547-552. [PubMed] [Google Scholar]
- 4.Anonymous. 2004. Update: influenza activity—United States, 2003-04 season. MMWR Morb. Mortal. Wkly. Rep. 53:284-287. [PubMed] [Google Scholar]
- 5.Banks, J., E. Speidel, and D. J. Alexander. 1998. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch. Virol. 143:781-787. [DOI] [PubMed] [Google Scholar]
- 6.Banks, J., E. C. Speidel, J. W. McCauley, and D. J. Alexander. 2000. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch. Virol. 145:1047-1058. [DOI] [PubMed] [Google Scholar]
- 7.Belser, J. A., O. Blixt, L. M. Chen, C. Pappas, T. R. Maines, N. Van Hoeven, R. Donis, J. Busch, R. McBride, J. C. Paulson, J. M. Katz, and T. M. Tumpey. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl. Acad. Sci. U. S. A. 105:7558-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch, F. X., W. Garten, H. D. Klenk, and R. Rott. 1981. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of avian influenza viruses. Virology 113:725-735. [DOI] [PubMed] [Google Scholar]
- 9.Capua, I., and S. Marangon. 2004. Vaccination for avian influenza in Asia. Vaccine 22:4137-4138. [DOI] [PubMed] [Google Scholar]
- 10.Chen, H., Y. Matsuoka, D. Swayne, Q. Chen, N. J. Cox, B. R. Murphy, and K. Subbarao. 2003. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine 21:4430-4436. [DOI] [PubMed] [Google Scholar]
- 11.Chen, H., K. Subbarao, D. Swayne, Q. Chen, X. Lu, J. Katz, N. Cox, and Y. Matsuoka. 2003. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine 21:1974-1979. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J., Y. F. Lau, E. W. Lamirande, C. D. Paddock, J. H. Bartlett, S. R. Zaki, and K. Subbarao. 2010. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 84:1289-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox, R. J., A. S. Madhun, S. Hauge, H. Sjursen, D. Major, M. Kuhne, K. Hoschler, M. Saville, F. R. Vogel, W. Barclay, I. Donatelli, M. Zambon, J. Wood, and L. R. Haaheim. 2009. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 27:1889-1897. [DOI] [PubMed] [Google Scholar]
- 14.de Wit, E., V. Munster, M. I. J. Spronken, T. M. Bestebroer, C. Baas, W. E. P. Beyer, G. F. Rimmelzwaan, A. D. M. E. Osterhaus, and R. A. M. Fouchier. 2005. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 79:12401-12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein, S. L., C. Y. Lo, J. A. Misplon, C. M. Lawson, B. A. Hendrickson, E. E. Max, and K. Subbarao. 1997. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J. Immunol. 158:1222-1230. [PubMed] [Google Scholar]
- 16.Fouchier, R. A., G. F. Rimmelzwaan, T. Kuiken, and A. D. Osterhaus. 2005. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr. Opin. Infect. Dis. 18:141-146. [DOI] [PubMed] [Google Scholar]
- 17.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann, E., G. Neumann, Y. Kawaoka, G. Hobom, and R. G. Webster. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. U. S. A. 97:6108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadhao, S. J., J. Achenbach, D. E. Swayne, R. Donis, N. Cox, and Y. Matsuoka. 2008. Development of Eurasian H7N7/PR8 high growth reassortant virus for clinical evaluation as an inactivated pandemic influenza vaccine. Vaccine 26:1742-1750. [DOI] [PubMed] [Google Scholar]
- 20.Jin, H., B. Lu, H. Zhou, C. Ma, J. Zhao, C. F. Yang, G. Kemble, and H. Greenberg. 2003. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306:18-24. [DOI] [PubMed] [Google Scholar]
- 21.Jin, H., H. Zhou, B. Lu, and G. Kemble. 2004. Imparting temperature sensitivity and attenuation in ferrets to A/Puerto Rico/8/34 influenza virus by transferring the genetic signature for temperature sensitivity from cold-adapted A/Ann Arbor/6/60. J. Virol. 78:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph, T., J. McAuliffe, B. Lu, H. Jin, G. Kemble, and K. Subbarao. 2007. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J. Virol. 81:10558-10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph, T., J. McAuliffe, B. Lu, L. Vogel, D. Swayne, H. Jin, G. Kemble, and K. Subbarao. 2008. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology 378:123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karron, R. A., K. Callahan, C. Luke, B. Thumar, J. McAuliffe, E. Schappell, T. Joseph, K. Coelingh, H. Jin, G. Kemble, B. R. Murphy, and K. Subbarao. 2009. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J. Infect. Dis. 199:711-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karron, R. A., K. Talaat, C. Luke, K. Callahan, B. Thumar, S. Dilorenzo, J. McAuliffe, E. Schappell, A. Suguitan, K. Mills, G. Chen, E. Lamirande, K. Coelingh, H. Jin, B. R. Murphy, G. Kemble, and K. Subbarao. 2009. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 27:4953-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopmans, M., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R. Fouchier, A. Osterhaus, and A. Bosman. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587-593. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz, J., R. J. Manvell, and J. Banks. 1996. Avian influenza virus isolated from a woman with conjunctivitis. Lancet 348:901-902. [DOI] [PubMed] [Google Scholar]
- 28.Luke, C. J., and K. Subbarao. 2006. Vaccines for pandemic influenza. Emerg. Infect. Dis. 12:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappas, C., Y. Matsuoka, D. E. Swayne, and R. O. Donis. 2007. Development and evaluation of an influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin. Vaccine Immunol. 14:1425-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasick, J., K. Handel, J. Robinson, J. Copps, D. Ridd, K. Hills, H. Kehler, C. Cottam-Birt, J. Neufeld, Y. Berhane, and S. Czub. 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J. Gen. Virol. 86:727-731. [DOI] [PubMed] [Google Scholar]
- 31.Perdue, M. L., M. Garcia, D. Senne, and M. Fraire. 1997. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 49:173-186. [DOI] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Spackman, E., D. A. Senne, S. Davison, and D. L. Suarez. 2003. Sequence analysis of recent H7 avian influenza viruses associated with three different outbreaks in commercial poultry in the United States. J. Virol. 77:13399-13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suarez, D. L., D. A. Senne, J. Banks, I. H. Brown, S. C. Essen, C. W. Lee, R. J. Manvell, C. Mathieu-Benson, V. Moreno, J. C. Pedersen, B. Panigrahy, H. Rojas, E. Spackman, and D. J. Alexander. 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 10:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbarao, K., and T. Joseph. 2007. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 7:267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suguitan, A. L., Jr., M. P. Marino, P. D. Desai, L. M. Chen, Y. Matsuoka, R. O. Donis, H. Jin, D. E. Swayne, G. Kemble, and K. Subbarao. 2009. The influence of the multi-basic cleavage site of the H5 hemagglutinin on the attenuation, immunogenicity and efficacy of a live attenuated influenza A H5N1 cold-adapted vaccine virus. Virology 395:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suguitan, A. L., Jr., J. McAuliffe, K. L. Mills, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swayne, D. E., and R. D. Slemons. 1994. Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation. Vet. Pathol. 31:237-245. [DOI] [PubMed] [Google Scholar]
- 39.Talaat, K. R., R. A. Karron, K. A. Callahan, C. J. Luke, S. C. DiLorenzo, G. L. Chen, E. W. Lamirande, H. Jin, K. L. Coelingh, B. R. Murphy, G. Kemble, and K. Subbarao. 2009. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine 27:3744-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tweed, S. A., D. M. Skowronski, S. T. David, A. Larder, M. Petric, W. Lees, Y. Li, J. Katz, M. Krajden, R. Tellier, C. Halpert, M. Hirst, C. Astell, D. Lawrence, and A. Mak. 2004. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10:2196-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster, R. G., and R. Rott. 1987. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell 50:665-666. [DOI] [PubMed] [Google Scholar]
- 42.Whiteley, A., D. Major, I. Legastelois, L. Campitelli, I. Donatelli, C. I. Thompson, M. C. Zambon, J. M. Wood, and W. S. Barclay. 2007. Generation of candidate human influenza vaccine strains in cell culture—rehearsing the European response to an H7N1 pandemic threat. Influenza Other Respi. Viruses 1:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]