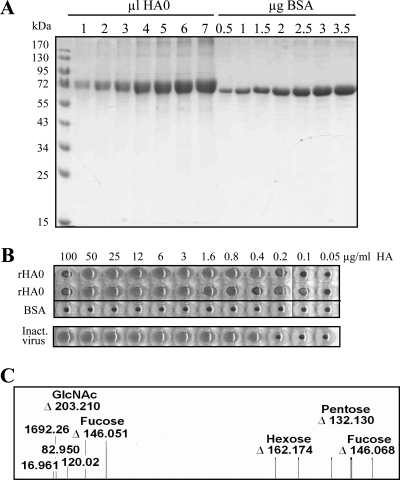
FIG. 3.
Analysis of purified rHA0 for estimations of concentration and purity. (A) SDS-PAGE. Aliquots of rHA0 (1, 2, 3, 4, 5, 6, and 7 μl) as well as aliquots of bovine serum albumin (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 μg) were resolved on a 12% polyacrylamide gel and stained with Coomassie. (B) Hemagglutination assay of purified rHA0. Twofold serial dilutions of rHA0 starting with a concentration of 400 μg/ml were tested for hemagglutination activity. Twofold dilutions of inactivated NIBRG-14 virus (code 05/204; NIBSC, United Kingdom) starting with a concentration of 28 μg/ml were used as a positive control. Twofold dilutions of bovine serum albumin starting with a concentration of 400 μg/ml were used as a negative control. All sample dilutions were performed in duplicate. (C) Selected part of a processed LID-LIFT spectrum of a 3,385.570-Da glycopeptide (amino acid residues 480 to 493) from tryptic digestion of rHA0. The peptide part of this glycopeptide is 1,692.26 Da.