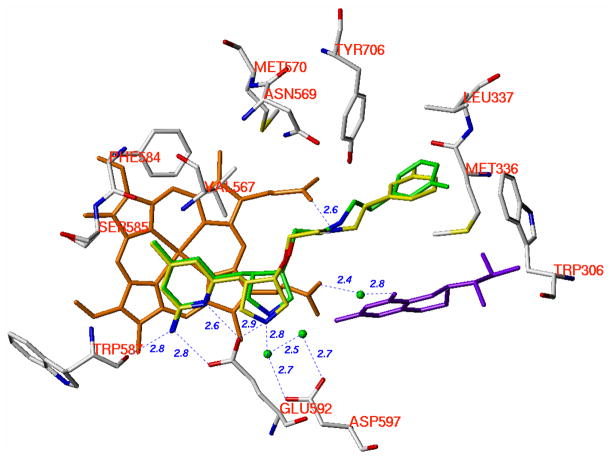
Figure 7.
Superimposition of the crystallographic binding conformations of (3′R, 4′S)-4 and (3′S, 4′S)-4. The heme (orange), H4B (purple), and structural water (green) involved in the binding of inhibitor to nNOS are shown. The active site residues are represented in an atom-type style (carbons in grey, nitrogens in blue, oxygen in red, and sulfur in yellow). The carbons of (3′R, 4′S)-4 are colored green, and the carbons of (3′S, 4′S)-4 are colored yellow. The nitrogens and the fluorine are colored blue and green, respectively. The distances of the H-bonds between the residues, structural water, cofactors and inhibitors from the crystal structure of nNOS in a complex with (3′S, 4′S)-4 are indicated in Angstroms (Å). The corresponding distances from the crystal structure of nNOS in a complex with (3′R, 4′S)-4 are similar but not shown.