Figure 3.
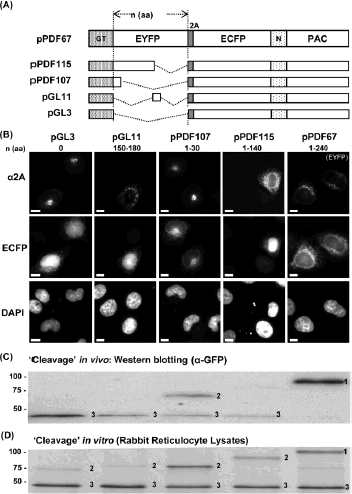
Effect of inclusion of a nuclear localisation signal. To visualise the partitioning of ECFP between the cytoplasm and the ER, we included a nuclear localisation signal (N; together with PAC) such that any cytoplas-mic [ECFP-N-PAC] would be localised in the nucleus (panel A). The number of residues between the end of the transmembrane domain of the GT signal sequence and the N-terminal residue of 2A are shown above the appropriate images in panel B. HeLa cells were transfected with plasmid constructs and images taken 24 h later (scale bar = 10 μm). The localisation of EYFP and ECFP was determined by their native fluorescence, whilst the localisation of the deletion forms of EYFP was determined using anti-2A antibodies (panel B). Cell lysates were prepared, separated by SDS–PAGE and subsequently Western blotted using anti-GFP antibodies (panel C). Rabbit reticulocyte in vitro translation systems were programmed with constructs as indicated and the incorporation of 35S-methionine determined by SDS–PAGE and phosphorimaging. Translation products are denoted as; 1 = [GT-EYFP-2A-ECFP-N-PAC], 2 = [GT-ΔEYFP-2A-ECFP-N-PAC] and 3 = [ECFP-N-PAC]. Note: the gel system we used was designed to resolve the [GT-ΔEYFP-2A-ECFP-N-PAC]/[GT-EYFP-2A-ECFP-N-PAC] products from [ECFP-N-PAC], and the [GT-ΔEYFP-2A] products from pPDF115/pPDF107/pGL1.The[GT-2A] product from pGL3 were not resolved in this system. Lanes in PAGE analyses of proteins (panels C and D) were derived from constructs used to produce the cell images shown in panel B, above.
