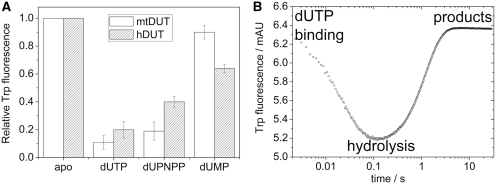
Figure 5.
The Trp fluorescence of hDUTF158W and mtDUTH145W reflects the relative energy changes of the π–π interaction during the enzymatic cycle. (A) Maximal fluorescence changes upon substrate or product binding to the Trp-bearing active site of mtDUTH145W (white bars) and of hDUTF158W (grey bars). The aromatic stacking between the uracil ring of the ligand and the Trp residue conveys a characteristic fluorescence quench with similar ligand-dependent tendency in both enzymes [human dUTPase data from (23)]. The largest quench is observed in an actively cycling enzyme (i.e. in the presence of dUTP). (B) Fluorescence changes on a rapid logarithmic time base in a STO stopped-flow experiment presented above. Similar fluorescence behavior has been reported for the human dUTPase (23), hence we only show that of the mtDUTH145W here. Upon mixing dUTPase with dUTP, a large and rapid fluorescence quench is observed which reflect substrate binding and subsequent conformational changes of the active site. Fluorescence then recovers to its near starting level (depending on the concentration of dUMP present in the solution). The rate constant of fluorescence recovery, i.e. the exit from the low fluorescence conformational state, is identical in a STO case with that of the hydrolysis step (23).