Abstract
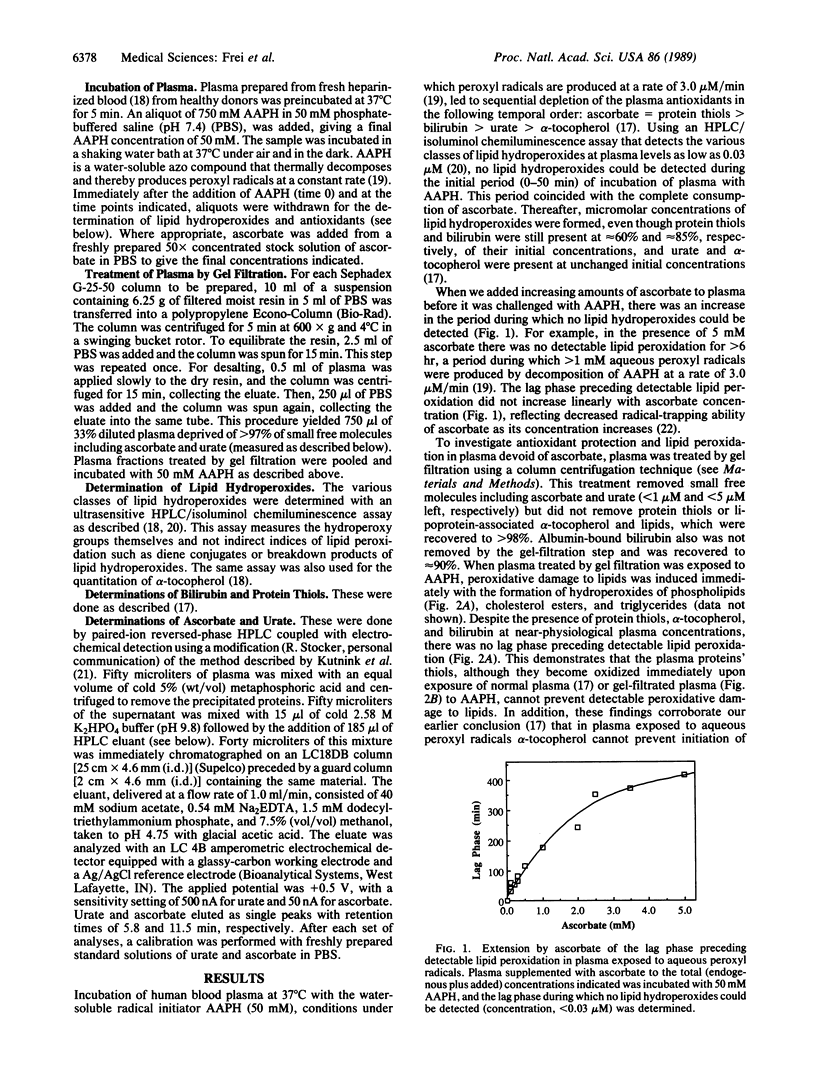
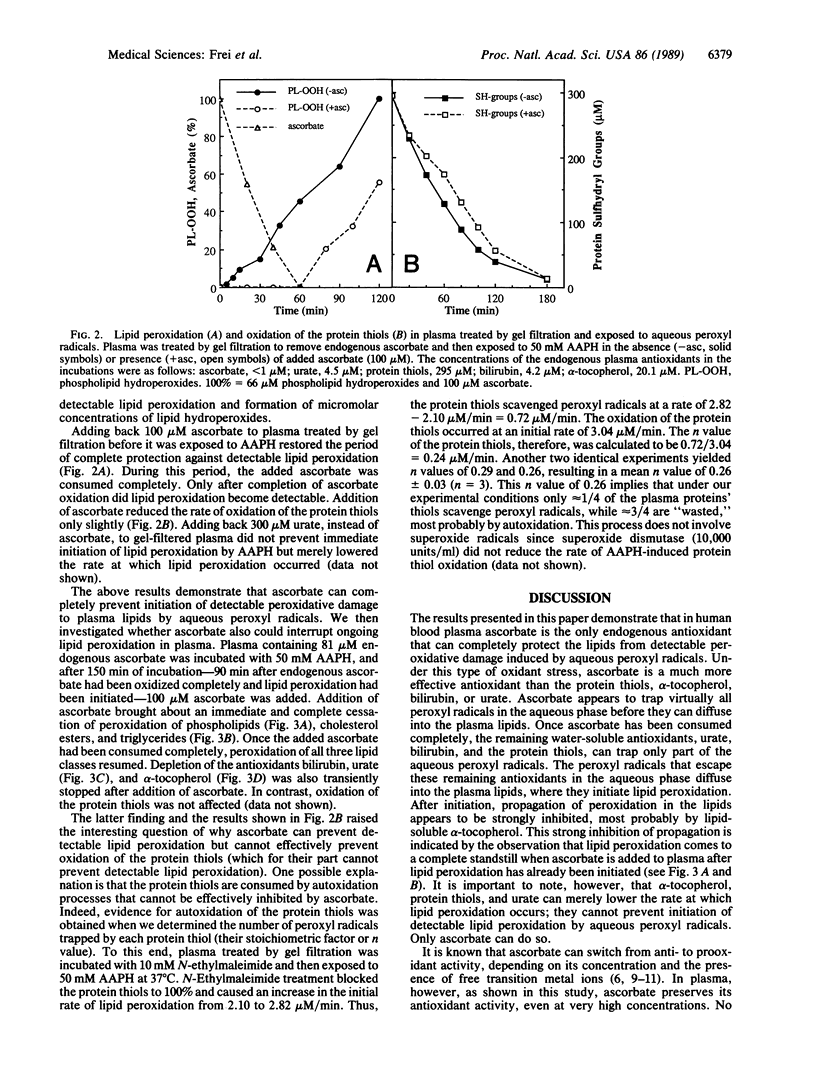
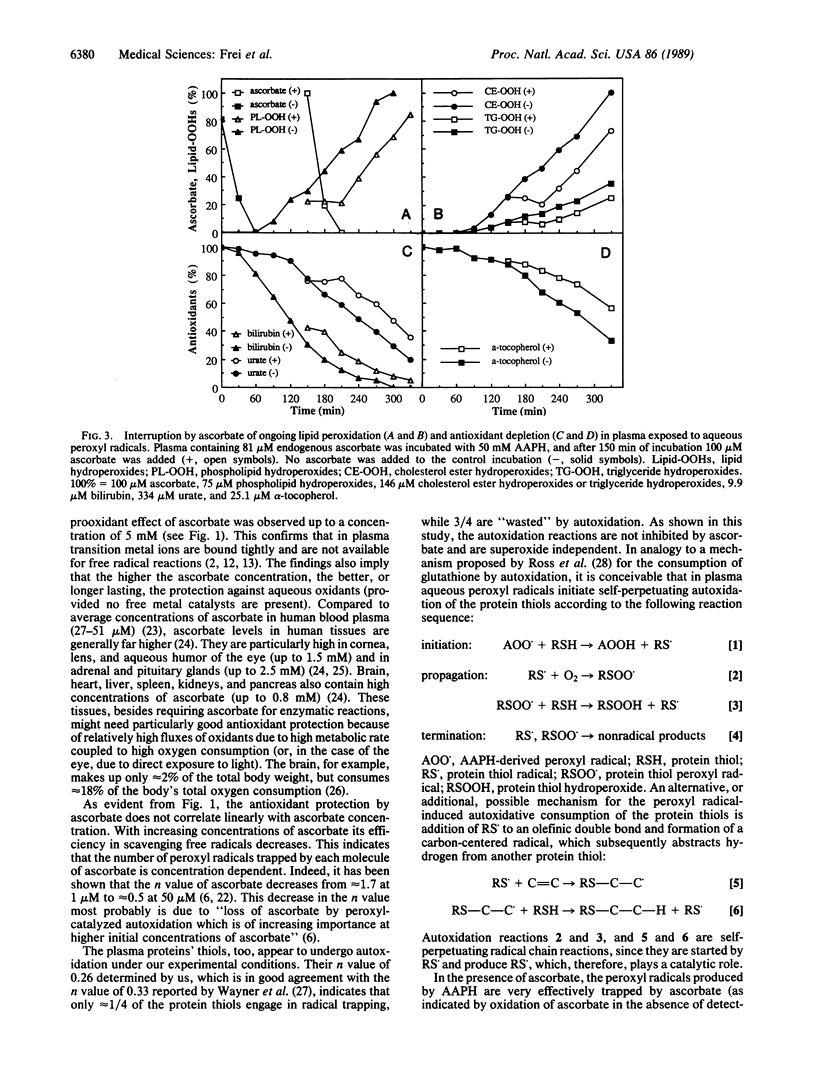
We have shown recently that the temporal order of antioxidant consumption in human blood plasma exposed to a constant flux of aqueous peroxyl radicals is ascorbate = protein thiols greater than bilirubin greater than urate greater than alpha-tocopherol and that detectable lipid peroxidation starts only after ascorbate has been consumed completely. In this paper, we show that it is indeed ascorbate that completely protects plasma lipids against detectable peroxidative damage induced by aqueous peroxyl radicals and that ascorbate is the only plasma antioxidant that can do so. Plasma devoid of ascorbate, but no other endogenous antioxidant, is extremely vulnerable to oxidant stress and susceptible to peroxidative damage to lipids. The plasma proteins' thiols, although they become oxidized immediately upon exposure to aqueous peroxyl radicals, are inefficient radical scavengers and appear to be consumed mainly by autoxidation. Our data demonstrate that ascorbate is the most effective aqueous-phase antioxidant in human blood plasma and suggest that in humans ascorbate is a physiological antioxidant of major importance for protection against diseases and degenerative processes caused by oxidant stress.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Barber A. A. Lipid peroxidation in rat tissue homogenates: Interaction of iron and ascorbic acid as the normal catalytic mechanism. Lipids. 1966 Mar;1(2):146–151. doi: 10.1007/BF02533008. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Yamamoto Y., Niclas D., Ames B. N. Evaluation of an isoluminol chemiluminescence assay for the detection of hydroperoxides in human blood plasma. Anal Biochem. 1988 Nov 15;175(1):120–130. doi: 10.1016/0003-2697(88)90369-7. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975 Sep 30;258:103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- Jacob R. A., Skala J. H., Omaye S. T. Biochemical indices of human vitamin C status. Am J Clin Nutr. 1987 Nov;46(5):818–826. doi: 10.1093/ajcn/46.5.818. [DOI] [PubMed] [Google Scholar]
- Kutnink M. A., Hawkes W. C., Schaus E. E., Omaye S. T. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987 Nov 1;166(2):424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986 Apr 3;314(14):892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- Ross D., Norbeck K., Moldéus P. The generation and subsequent fate of glutathionyl radicals in biological systems. J Biol Chem. 1985 Dec 5;260(28):15028–15032. [PubMed] [Google Scholar]
- Sadrzadeh S. M., Eaton J. W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Invest. 1988 Nov;82(5):1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Weidemann M. J., Hunt N. H. Possible mechanisms responsible for the increased ascorbic acid content of Plasmodium vinckei-infected mouse erythrocytes. Biochim Biophys Acta. 1986 May 2;881(3):391–397. doi: 10.1016/0304-4165(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Barclay L. R., Locke S. J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987 Jun 22;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U. The antioxidant efficiency of vitamin C is concentration-dependent. Biochim Biophys Acta. 1986 Oct 29;884(1):119–123. doi: 10.1016/0304-4165(86)90234-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Niki E. Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochim Biophys Acta. 1988 Jan 19;958(1):19–23. doi: 10.1016/0005-2760(88)90241-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]


