Table 1.
Optimization of the Michael reaction.
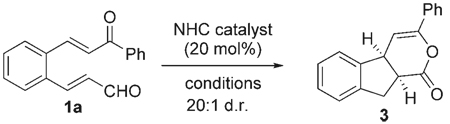 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Conditions[a] | Yield[b] | ee [%][c] |
| 1 | A | DBU, THF | 6 | – |
| 2 | A | KN(SiMe3)2, THF[d] | 11 | – |
| 3 | A | iPr2EtN, THF, 45°C | 50 | – |
| 4 | B | iPr2EtN, toluene/THF[e] | 61 | – |
| 5 | C | iPr2EtN, toluene/THF[e,f] | 61 | 93 |
| 6 | C | Et3N (0.1 m), toluene/THF[e] | 62 | 93 |
| 7 | C | iPr2EtN (0.1 m), CH2Cl2, −20°C | 49 | 93 |
| 8 | D | iPr2EtN (0.1 m), toluene/THF[e] | 53 | 99 |
| 9 | D | iPr2EtN (0.05 m), toluene/THF[e] | 66 | 99 |
| 10 | D | iPr2EtN (0.05 m), CH2Cl2 | 68 | 99 |
| 11 | D[g] | iPr2EtN (0.05 m), CH2Cl2 | 68 | 99 |
Base (20 mol %), 1a (0.2 m) at 23°C unless otherwise noted. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene.
Yield of isolated product.
Determined by HPLC (Chiracel AD-H). Absolute and relative configuration of 3 assigned by X-ray crystallography.[16] See the Supporting Information for details.
Carbene generated prior to addition of substrate.
10:1 toluene/THF.
Base (1.2 equiv).
D (10 mol %). ![[g]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/f179/2978499/db6e8c90974d/nihms248707t2.jpg)
