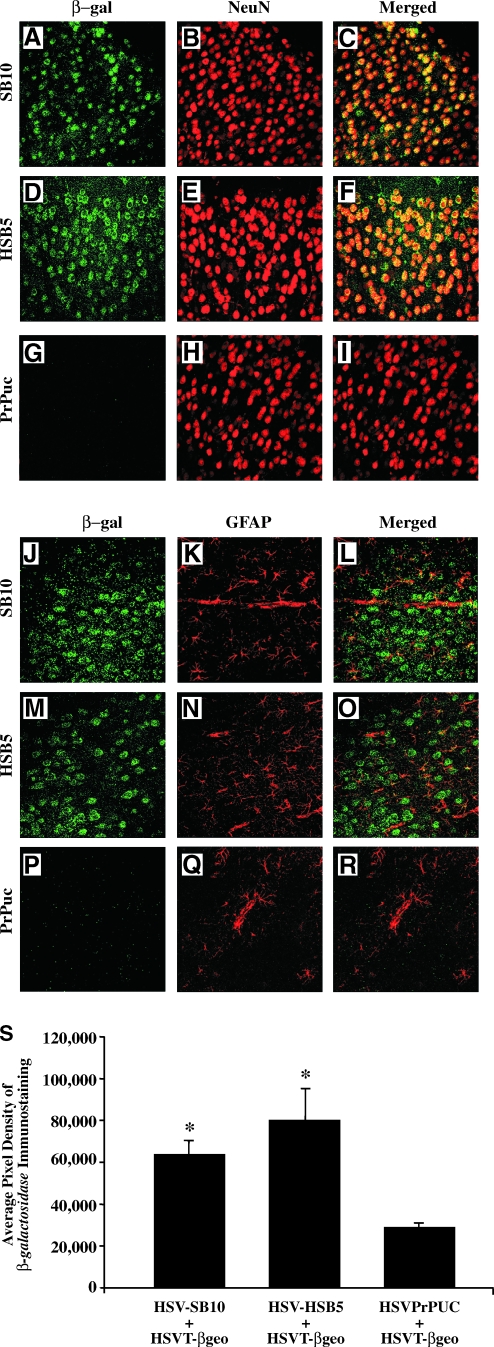
FIG. 6.
Immunohistochemical analysis and quantification of the cell type-specific expression of the T-βgeo transgenon (i.e., a transposable transgene) on P21 after in utero delivery. At 21 days of age, transduced animals were killed and perfused with 4% paraformaldehyde, brain sections were processed for dual β-galactosidase (β-Gal)/NeuN immunohistochemistry, and sections were imaged by confocal microscopy. Representative brain sections corresponding to the cortex are depicted. β-Gal-specific staining resulting from T-βgeo transgenon-mediated expression appears in the green channel (A, D, and G), NeuN-positive mature neurons in the red channel (B, E, and H), and colocalized staining (Merged) appears as yellow (C, F, and I). Similarly, brain sections were processed for dual β-Gal/GFAP immunohistochemistry (J–R). The GFAP-positive astrocytes appear in the red channel. Photomicrographs were obtained at an original magnification of ×40. Scale bar (A): 200 μm. β-Gal-specific diaminobenzidine (DAB) immunostaining was conducted on coronal brain sections of the left hemisphere to quantify the average pixel density of β-galactosidase-positive neurons in the visual motor cortex (VM) region of mice that were coinjected with HSV-SB10, HSV-HSB5, or HSVPrPUC along with the HSVT-βgeo amplicon (S). β-Gal-positive cells in the VM region were enumerated with an Olympus AX-70 microscope equipped with a motorized stage and MCID 6.0 Elite software. An average of eight equivalent sections of the VM region per mouse were analyzed at a magnification of × 20. Error bars represent the standard error of the mean and statistical analysis was conducted by Student t test. *Statistically significant difference between the two HSV-SB amplicon (SB10 and HSB5) plus HSVT-βgeo-injected groups and the HSVPrPUC plus HSVT-βgeo control group (p < 0.05).