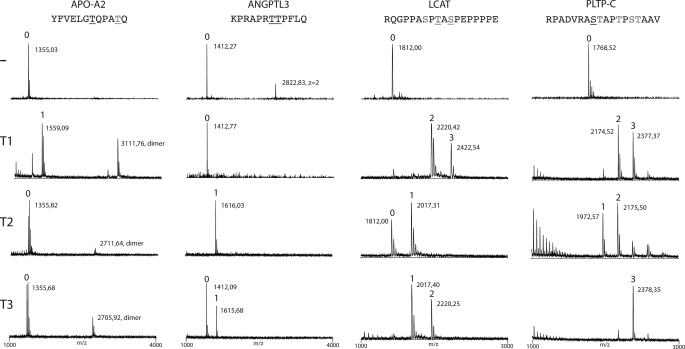
FIGURE 1.
ANGPTL3 is selectively O-glycosylated by GalNAc-T2. In vitro screening of substrate specificities of recombinant human GalNAc-transferases, GalNAc-T1, -T2, and -T3, with peptides covering potential O-glycosylation sites identified in four proteins involved in HDL and TG metabolism (APO-A2, ANGPTL3, LCAT, and PLTP-C) (Table 1). Glycosylation of peptides was monitored by MALDI-TOF analysis, and products formed after 24 h incubation with enzymes are shown. Masses of peptides and glycopeptides are indicated with the predicted number of incorporated GalNAc residues indicated above the peaks. APO-A2 was only glycosylated by GalNAc-T1 (one GalNAc residue), ANGPTL3 was glycosylated by GalNAc-T2 (one GalNAc residue) and only partially by GalNAc-T3, and LCAT and PLTP-C were glycosylated by all three tested GalNAc-transferases with a varying number of GalNAc residues incorporated. Sequences of peptide substrates shown above with potential Ser and Thr O-glycosylation sites indicated by underlining with black type, sites predicted by the NetOGlyc server are shown with gray type, and sites experimentally verified are shown with underlining with gray type. The APO-A2 peptide partly formed dimers as indicated.