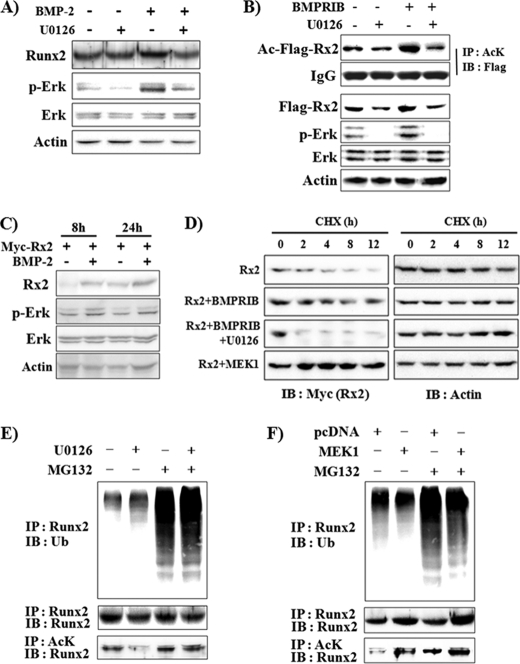
FIGURE 2.
Erk activation increases Runx2 acetylation and stability, whereas it decreases Runx2 ubiquitination. A, U0126 decreased BMP-2-induced Runx2 protein expression. C2C12 cells were pretreated with vehicle or U0126 for 1 h and further incubated in the presence or absence of BMP-2 (200 ng/ml) for an additional 48 h. Endogenous Runx2 protein levels were determined by immunoprecipitation and immunoblot analysis. B, U0126 suppressed constitutively active BMP type IB (BMPR-IB)-induced increase in Runx2 protein levels and acetylation. C2C12 cells were transiently transfected with FLAG-Runx2 and BMPR-IB expression plasmids and incubated for 24 h. When indicated, the cells were incubated with U0126 for the last 3 h. Immunoprecipitation (IP) and immunoblot (IB) analysis were then performed. AcK, anti-acetylated lysine antibody. C, BMP-2 increased exogenously expressed Myc-Runx2 protein levels. C2C12 cells were transfected with Myc-Runx2, incubated for 8 or 24 h in the presence of BMP-2, and subjected to IB analysis. D, Erk activation by BMPR-IB or MEK1 expression stabilized Runx2 protein, whereas U0126 enhanced degradation of Runx2. 293T cells were transiently transfected with Myc-Runx2 and concomitantly with either BMPR-IB or MEK1 as indicated. Thirty hours after transfection the cells were treated with cycloheximide (CHX, 10 μg/ml) in the presence or absence of U0126 for the indicated times. The levels of Myc-Runx2 and actin were determined by IB analysis. E and F, U0126 increased, whereas Erk activation decreased Runx2 ubiquitination (upper panel). C2C12 cells were treated with vehicle or 5 μm MG132 for 18 h. When indicated, U0126 was added for the last 3 h (E). C2C12 cells were transfected with a MEK1 expression vector, incubated for 24 h, and treated with vehicle or MG132 for an additional 18 h (F). Ubiquitinated endogenous Runx2 was detected by IP with an anti-Runx2 antibody and subsequent IB with an anti-ubiquitin (Ub) antibody. Nonubiquitinated Runx2 and acetylated Runx2 were also examined using the same cell lysates (middle and lower panels).