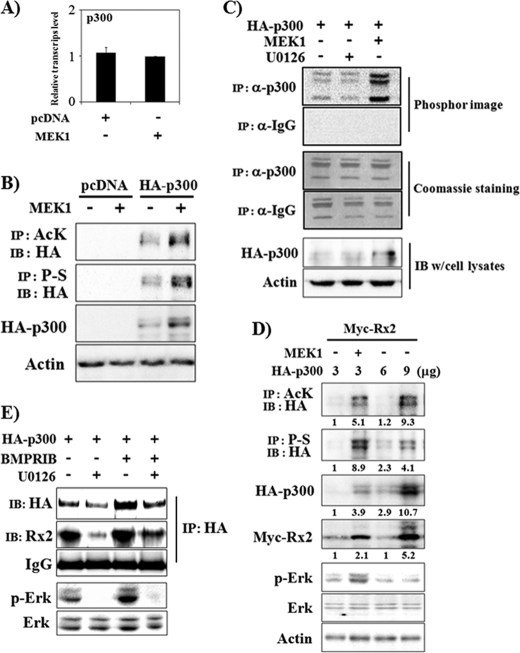
FIGURE 4.
Erk activation increases protein levels and HAT activity of p300 and the association of Runx2 and p300. A, Erk activation did not significantly increase p300 mRNA levels. C2C12 cells were transiently transfected with a MEK1 expression vector and incubated for 13 h. Total RNA was then prepared, and real-time PCR was performed. B and C, Erk activation increases p300 protein levels and HAT activity. 293T cells were transiently transfected with HA-p300 and MEK1. Forty-eight hours after transfection, whole cell lysates were prepared for IP and/or IB analysis. When indicated, U0126 was used for the last 2 h (C). AcK, anti-acetylated lysine antibody. A HAT assay was performed using immunoprecipitates with anti-p300 antibody or with control IgG, and 14C-labeled acetylated histone levels were detected by phosphor-imaging (C, upper panel). Coomassie staining shows equal loading of histone mixture used for HAT assay (C, middle panel), and IB results represent HA-p300 expression levels of cell lysates (C, lower panel). D, Erk activation increased p300 acetylation, phosphorylation, and protein levels. The Runx2 protein level was related to the acetylated p300 protein level. 293T cells were transiently transfected with Myc-Runx2 and increasing amounts of HA-p300 expression vector. When indicated, MEK1 was also overexpressed. Forty-eight hours after transfection, whole cell lysates were prepared for IP and/or IB analysis. Densitometry analysis results were provided under the respective bands. P-S, anti-phosphoserine antibody. E, U0126 suppressed the physical association between Runx2 and p300. C2C12 cells were transiently transfected with HA-p300 and BMPR-IB expression vectors and incubated in the presence or absence of U0126 for 48 h. Whole cell lysates were then prepared, and IP and/or IB analysis was performed.