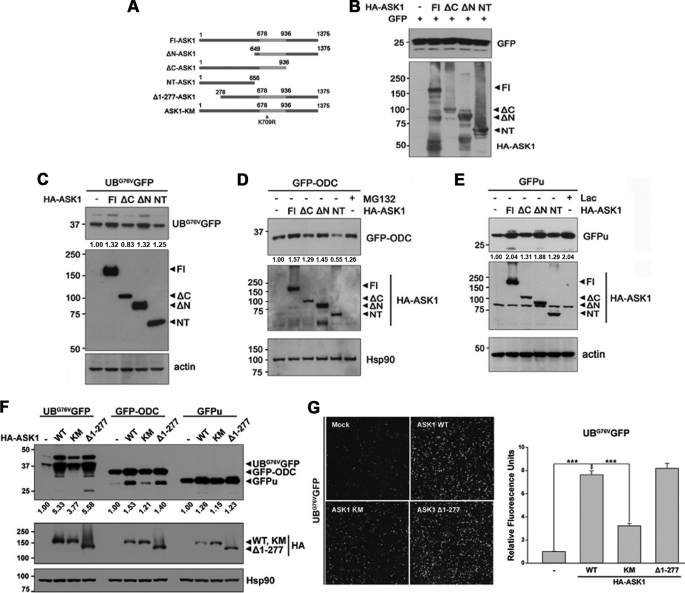
FIGURE 2.
The C-terminal domain of ASK1 is critical for the down-regulation of proteasome function. A, schematic diagram of full-length ASK1 (Fl-ASK1), truncation mutants, and catalytically inactive mutant. The ASK1-kinase domain located at 676–936 amino acids is marked in gray. B–E, HEK293 cells were transfected with HA-tagged full-length ASK1 (Fl) or ASK1 deletion mutants (ΔC-ASK1, ΔN-ASK1, and NT-ASK1) along with GFP vector (B), UbG76VGFP (C), GFP-ODC (D), or GFPu (E). Total cell lysates were probed with anti-GFP or anti-HA antibodies. Actin or Hsp90 served as a loading control. The values at the bottom of the top panel indicate the relative intensities of UbG76VGFP (C), GFP-ODC (D), or GFPu (E) bands measured using MultiGauge version 3.1. Lac, lactacystin. F, HEK293 cells were transfected with HA-tagged full-length ASK1 (Fl), its kinase-inactive K709R mutant (ASK1-KM), or constitutive active mutant (Δ1–277-ASK1) along with UbG76VGFP, GFP-ODC, or GFPu. Total cell lysates were probed with anti-GFP or anti-HA antibodies. Western blots are representative of three independent experiments. Hsp90 served as a loading control. The values at the bottom of the top panel indicate the relative intensities of UbG76VGFP, GFPu, or GFP-ODC bands measured using MultiGauge version 3.1. Western blot images and quantification values in B–F are representative of three independent experiments. G, after HEK293 cells were transfected with HA-tagged full-length ASK1, its kinase-inactive K709R mutant (ASK1-KM), or constitutive active mutant (Δ1–277-ASK1) along with UbG76VGFP, UbG76VGFP degradation was observed by fluorescent microscopy (left) or measured at λEX = 485/λEM = 535 using a VICTOR X3 (***, p < 0.001) (right).