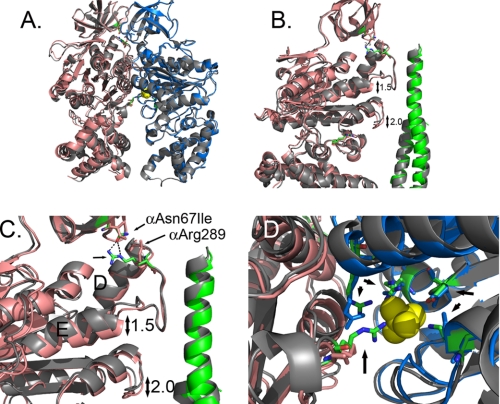
FIGURE 2.
Structural changes observed in the F1 structure with mgi mutation, αAsn67Ile. A shows an overlap of the E site α- and β-subunits form the wild type (gray) and αAsn67Ile structure. The α- and β-subunits of the mutant structure are shown in salmon and blue, respectively. B is a closer view showing the shifts in the position of the helix following the collar region and the region in the α-subunit that is the structural analog to the Catch 1 region of the β-subunit. The wild-type γ-subunit is colored gray, and the mutant structure is colored green. C shows the disruption of the H-bond between αAsn67 and αArg289 with the αAsn67Ile mutation. The residues are shown in stick representation with the wild type colored as defined by the atom. D shows a view of the phosphate binding site and the relative changes in the atom positions with phosphate shown in yellow. The atoms of the wild-type structure are colored as defined by the atom. The residues indicated by the arrows correspond to: αArg375, βArg190, βArg260, βAsp256, and βLys163 (P-loop) starting at the bottom and proceeding in a clockwise direction.