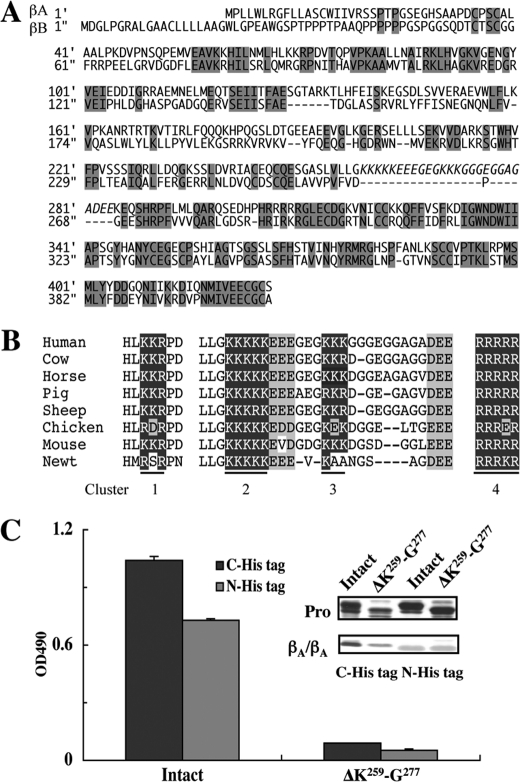
FIGURE 6.
Abrogation of the perlecan binding activity of activin A by deletion of the inhibin βA subunit-specific region harboring Clusters 2 and 3. A, amino acid sequence alignment between the inhibin βA- and βB-subunits. The peptide segment Lys259-Glu284 containing Clusters 2 and 3 in the inhibin βA-subunit is absent from the inhibin βB-subunit (highlighted in italics). The conserved amino acid residues are shaded in gray. B, alignment of basic amino acid clusters in the amino acid sequences of the inhibin βA-subunits from different species. The amino acid sequence of the peptide segment Lys259-Glu284 harboring Clusters 2 and 3 is well conserved among the different species. The clusters of basic amino acid residues are shown in white letters on black whereas acidic amino acid residues are shaded in gray. C, the perlecan binding activities of recombinant activin A with a His6 tag at either the N terminus (N-His tag) or C terminus (C-His tag) and those with deletion of the Lys259-Gly277 segment (designated ΔK259-G277) were determined by solid-phase binding assays. The SDS-PAGE profiles of purified activin A with and without deletion of Clusters 2 and 3 are shown in the inset. Note that the pro-regions of intact and mutant activin A differ in their sizes because of the presence or absence of the Lys259-Gly277 segment.