Abstract
The U21 open reading frame from human herpesvirus-7 encodes a membrane protein that associates with and redirects class I MHC molecules to the lysosomal compartment. The mechanism by which U21 accomplishes this trafficking excursion is unknown. Here we have examined the contribution of localization, glycosylation, domain structure, and the absence of substrate class I MHC molecules on the ability of U21 to traffic to lysosomes. Our results suggest the existence of a cellular protein necessary for U21-mediated rerouting of class I MHC molecules.
Keywords: Intracellular Trafficking, Lysosomes, Membrane Proteins, MHC, Trafficking, HHV-7, Viral Immune Evasion
Introduction
Human herpesvirus-6 and -7 (HHV-6 and -7)4 are β-herpesviruses most closely related to human cytomegalovirus. HHV-6 and -7 possess almost entirely collinear genomes and share many biological properties; both viruses infect T-lymphocytes, although they can infect other cell types as well, and both can cause high fever and exanthem subitum (roseola), although HHV-6 is the most common cause of this childhood disease (1, 2). Almost all of the population is seropositive for both viruses. Infection with HHV-6 occurs by the age of 2, whereas HHV-7 infection usually occurs slightly later (3). The cells infected with HHV-6 and -7 exhibit cytomegaly and are prone to syncytium formation, features reminiscent of those seen in human cytomegalovirus infection.
Like all other herpesviruses, HHV-6 and -7 remain latent or establish persistent infections. To do so, they must avoid detection and elimination by the immune system. Viral immune evasion strategies include restriction of viral gene expression, infection at immunoprivileged sites, obstruction of antiviral cytokine function, and interference with antigen presentation (for review see Ref. 4). Notably, all of the herpesviruses thus far examined employ the latter strategy of interfering with viral antigen presentation to cytotoxic T lymphocytes. Some herpesviral proteins interfere with proteolysis of antigens or peptide transport into the ER (5–7). Others retain class I molecules in the ER, mediate their destruction through ER-associated degradation, enhance the internalization of class I molecules, or divert class I molecules to lysosomes for degradation (8–17). Judging from the number and molecular diversity of these strategies, the removal of class I MHC-peptide complexes from the cell surface must be evolutionarily advantageous to these viruses as a means of escaping immune detection and thriving in a host.
Because so many of the viral immunoevasins affect trafficking or stability of class I MHC molecules, in previous work, we took a biochemical approach to examine the maturation and stability of class I molecules in HHV-7-infected T cells (11). We found that class I MHC stability was indeed decreased in HHV-7-infected T cells and that a 55-kDa viral glycoprotein coimmunoprecipitated with class I MHC molecules. We identified this associated protein as the product of the HHV-7 U21 open reading frame. HHV-7 U21 lacks homology to any other gene product or domain, with the exception of the U21 encoded by HHV-6. U21 is a type I membrane protein that binds to newly synthesized, properly folded MHC class I molecules in the ER, shortly after their synthesis. In previous studies, we expressed the U21 open reading frame in U373 astrocytoma cells, in the absence of viral context. Expression of U21 in U373 cells resulted in a dramatic redistribution of class I molecules to lysosomes and a commensurate reduction of class I molecules on the plasma membrane (see Fig. 2) (10, 11).
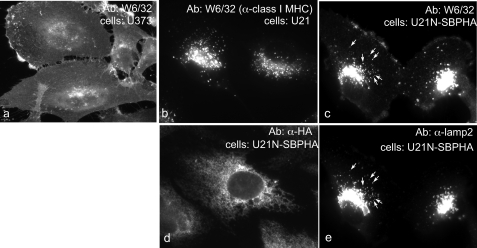
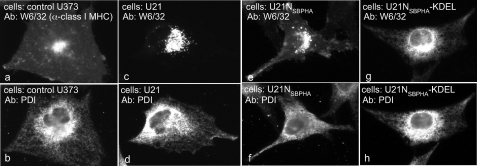
FIGURE 2.
Soluble U21NSBPHA diverts class I molecules intracellularly. Immunofluorescent detection of class I MHC (W6/32) (panels a, b, and c), U21NSBPHA (panel d), and lamp2 (panel e). The arrows denote points of colocalization.
We first hypothesized that the lumenal domain of U21 bound to class I MHC molecules, and the cytoplasmic tail of U21 contained the lysosomal targeting information necessary to reroute the two molecules to the lysosomal compartment. We tested this hypothesis by expressing a U21 molecule lacking its cytoplasmic tail in U373 cells. We found, to our surprise, that the cytoplasmic tail of U21 was not necessary for the ability of U21 to divert class I molecules to lysosomes (18). The tailless U21 molecule could divert class I MHC molecules to lysosomes, as could the lumenal domain of U21 fused to the transmembrane and the cytoplasmic tail of a heterologous type I membrane protein, CD4 (18). Thus, the lumenal domain of U21 is not only responsible for associating with class I molecules, but it also contains the information necessary to induce rerouting of class I MHC molecules to the lysosomal compartment. In the current study, we examine some of the molecular properties that govern U21-mediated diversion of class I MHC molecules to the lysosomal compartment.
EXPERIMENTAL PROCEDURES
Plasmids and shRNA Constructs
For the generation of β2-microglobulin (β2m) shRNA in human U373-MG cells, a 19-nucleotide region of the human β2m cDNA was cloned into pRETRO (19). The selected 19-nucleotide sequence is as follows (the numbers in parentheses indicate human β2m nucleotide coordinates, where 1 represents the adenosine of the start codon): TTGTGGAGCTTGTTGAATT (286–304). All cDNAs of U21 were cloned into the retroviral vectors pLNCX (Clontech), pHAGE-MCS (for K562 cells), or pHAGEpuroMCS (PPM). The lentiviral vector pHAGE was generously provided by Drs. Gustavo Mostoslavsky and Richard Mulligan (Harvard Medical School, Boston, MA) (19). The U21NSBPHA tandem tag construct consists of amino acids 1–358 of U21 followed by a streptavidin-binding protein tag and an HA epitope tag, separated by a tobacco etch virus protease cleavage site (SBPHA) (20). The U21NSBPHA KDEL construct contains an additional aaggatgaactgtga (KDEL) sequence following the HA portion of the tag. The HLA-A2-HA construct contains amino acids 1–336 of HLA-A2, followed by an HA tag and a stop codon. The murine H-2Kb chimera was constructed by fusing the cytoplasmic tail of HLA-A2 to the transmembrane region of H-2Kb. The amino acid sequence at the junction of the two molecules is as follows: VAVLVVLGAAIVTGAVVAFVRRRSSDRRGGSYSQAASSDSAQGSDVSLTACRV*, where the underline indicates the transmembrane sequence of H-2Kb, the plain type indicates the cytoplasmic tail of the HLA-A2 molecule, the bold type indicates the three lysines in the cytoplasmic tail of HLA-A2 that were mutated to arginines. The asterisk represents the stop codon.
Cell Lines
U373 astrocytoma cells were cultured in DMEM including 5% fetal bovine serum and 5% newborn calf serum in the presence or absence of puromycin (375 ng/ml) (Sigma-Aldrich, St. Louis, MO) or geneticin (500 μg/ml) (Invitrogen, Carlsbad, CA). Expression of the viral protein U21 and its variants in U373, HeLa, or K562 cells was carried out via retrovirus-mediated gene transfer. U373 astrocytoma cells were infected with retroviruses encoding HHV7-U21, a small hairpin RNA directed against β2m (shβ2m), U21NSBPHAKDEL, HHV-6A U21SBPHA, HHV-6B U21SBPHA, HHV-7 U21SBPHA, U21NSBPHA, HHV-6A U21HA, and the U21 glycosylation mutants. In most cases, the neomycin-resistant clones exhibited some heterogeneity of expression level, so clonal cell lines were analyzed, rather than pooled neomycin-resistant clones, so that cell populations appeared fairly uniform and are representative of >80% of the cells examined. The cell lines expressing HHV-7, -6A, and -6B U21SBPHA are described in Ref. 10. Cell lines expressing shβ2m were characterized and described in detail in Ref. 21. The myeloid K562 cells were spinoculated twice with a lentivirus encoding HHV7-U21.
Antibodies
W6/32 is a mAb that recognizes assembled, β2m-associated HLA-A, -B, or -C molecules. Anti-Giantin was purchased from Covance (San Diego, CA). Immunofluorescence studies were performed either with a polyclonal antibody (HA.11) directed against the HA epitope tag (Affiinity BioReagents, Golden, CO) or the anti-HA mAb 12CA5. Anti-HA immunoprecipitation experiments were performed with the 12CA5 mAb. Alexa Fluor 488- and 594-conjugated secondary antibodies were purchased from Molecular Probes (Eugene, OR). In some instances, Alexa 594-conjugated Fab fragments (Zenon; Invitrogen) were used to label lamp2 mAbs. Anti-lamp2 mAb was the generous gift of Dr. T. August (Johns Hopkins Medical School, Baltimore, MD). A phycoerythrin-conjugated anti-HLA-A,B,C mAb (BD Pharmingen, Franklin Lakes, NJ) was used in flow cytometry experiments. Polyclonal anti-protein-disulfide isomerase antiserum was generously supplied by Dr. H. Ploegh (Whitehead Institute, MIT, Boston, MA). The polyclonal antibody MCW50 is a rabbit polyclonal antibody that was raised against native GST fused in frame to the cytoplasmic tail of U21. The mAb Y3, directed against H-2Kb, was generously supplied by Dr. P. Cresswell (Yale University School of Medicine, New Haven, CT). The rabbit polyclonal antibody GM130 was purchased from BD Transduction Laboratories.
Pulse-Chase Experiments
The cells were detached with trypsin and incubated with methionine- and cysteine-free DMEM for 30 min at 37 °C. The cells were labeled with 350 μCi/ml of [35S]-Express label (1175Ci/mmol; PerkinElmer Life Sciences, Waltham, MA) at 37 °C for the indicated times and chased with complete DMEM supplemented with nonradiolabeled methionine and cysteine to a final concentration of 1 mm at 37 °C. The cells were lysed in Nonidet P-40 lysis buffer (50 mm Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 5 mm MgCl2, 150 mm NaCl). The lysates were centrifuged for 5–10 min at 16,000 × g at 4 °C in an Eppendorf centrifuge to pellet nuclei and debris, followed by immunoprecipitation with specific antiserum and protein A-agarose (RepliGen Corporation, Waltham, MA). Immunoprecipitates were normalized to equal trichloroacetic acid-precipitable 35S-labeled protein. The immunoprecipitates were washed five times with Nonidet P-40 wash buffer (50 mm Tris-HCl, pH 7.4, 0.5% Nonidet P-40, 5 mm EDTA, 150 mm NaCl) and subjected to SDS-PAGE. Leupeptin (200 μm) (EMD Biosciences, Inc., Gibbstown, NJ) and folimycin (20 nm) (concanamycin A) (EMD Biosciences, Inc.) were added to the cells 45 min prior to the pulse and were also included in the pulse and chase medium at the same concentrations. In some experiments, tunicamycin (Sigma) was added to the starvation medium at a final concentration of 10 μg/ml for 45 min and to the pulse and chase medium at the same final concentration. Peptide:N-glycosidase F (PNGase F) or and endoglycosidase Hf (New England Biolabs, Beverly, MA) were added to the immunoprecipitates according to the vendor's instructions.
Immunoblotting
The cells were washed and lysed as described above. The cell lysates were assayed for protein concentration in triplicate using the Pierce BCA protein assay kit (Thermo-Fisher, Rockford, IL). For immunoblotting of cell lysates, equal concentrations of protein from each sample was subjected to SDS-PAGE. The separated proteins were transferred to BA-85 nitrocellulose membrane (Whatman, Florham Park, NJ). Equal loading was further confirmed using Ponceau S staining of the nitrocellulose membrane. The membrane was simultaneously probed with HC10 (anti-class I MHC) and HA.11, followed by HRP-conjugated secondary antibody (Bio-Rad, Hercules, CA). The bands were visualized using the Pierce SuperSignal chemiluminescence reagents (Thermo-Fisher).
Immunofluorescence Microscopy
Immunofluorescence was performed as described (18) and followed by Alexa 488- or Alexa 594-conjugated secondary antibodies. Tunicamycin (New England Biolabs, Beverly, MA) was added to the cells at a final concentration of 5 μg/ml 10 h prior to fixation.
Flow Cytometry
Flow cytometry was performed as described (18), using phycoerythrin anti-human class I MHC (anti-HLA-A,B,C).
RESULTS
The Soluble Lumenal Domain of U21 Can Bind to and Reroute Class I Molecules to Lysosomes
To extend our knowledge of the ability of U21 to reroute class I MHC molecules to lysosomes, we next asked whether a soluble, secreted form of U21 could also divert class I molecules to lysosomes. To address this question, we generated a soluble form of U21 lacking a transmembrane domain (U21N), fused to a tandem affinity tag (SBPHA), comprised of a 46-amino acid streptavidin-binding protein domain and an HA epitope tag separated by a tobacco etch virus protease cleavage site (20). The entire tag, including spacer amino acids, is 78 amino acids long (Fig. 1a).
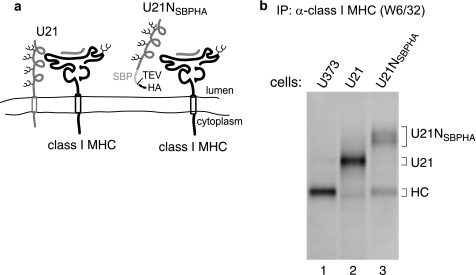
FIGURE 1.
W6/32 coimmunoprecipitates soluble U21NSBPHA-tagged molecules. a, hypothetical schematic depiction of U21 and class I MHC binding and depiction of the soluble U21NSBPHA molecule. b, U373, U21, and U21NSBPHA cells were labeled with [35S]methionine for 90 min, lysed, and recovered with W6/32, directed against properly folded class I MHC molecules. Migration positions of U21, U21NSBPHA, and class I heavy chain (HC) molecules are indicated. Note that this figure depicts a hypothetical interaction between U21 and class I molecules; interaction domains, if any, are unknown. IP, immunoprecipitation.
To determine whether the soluble U21NSBPHA molecule could associate with class I MHC molecules, we performed coimmunoprecipitation experiments from cells expressing U21NSBPHA. We recovered class I MHC molecules from U373 control cells, U21 cells, and U21NSBPHA cells with W6/32, an antibody directed against properly folded class I MHC molecules. In U21NSBPHA cells, a diffuse polypeptide of ∼65–75 kDa was recovered with class I molecules that is absent in immunoprecipitations from U373 control cells or U21-expressing cells (Fig. 1b, compare lane 3 with lanes 1 and 2). Immunoblotting confirmed the identity of this polypeptide as U21NSBPHA (data not shown). As would be predicted of a soluble U21NSBPHA molecule, it is secreted into the medium (data not shown), which, together with its ability to bind to class I MHC molecules, suggests that the soluble U21NSBPHA molecule is folded properly. In addition, soluble U21NSBPHA migrates diffusely in this SDS gel. We believe this diffuse migration to be due to complex-type oligosaccharide modifications that occur as the secreted U21NSBPHA molecule makes its way through the secretory pathway.
The 78-amino acid SBPHA tag increases the size of the U21 molecule by only 8 amino acids, because the chimera lacks its cytoplasmic tail (50 amino acids) and transmembrane domain (20 amino acids). Thus, U21NSBPHA should closely comigrate with U21. However, soluble U21NSBPHA exhibits aberrantly slow mobility in SDS-polyacrylamide gels (Fig. 1b, lane 3).
To determine whether the soluble U21NSBPHA could divert class I MHC molecules to the lysosomal compartment, we examined the localization of class I MHC molecules in cells expressing soluble U21NSBPHA (Fig. 2). For comparison, we show class I MHC localization on the cell surface in control U373 cells (Fig. 2a) and localization of class I MHC molecules in the lysosomal compartment in U21 cells (Fig. 2b). In cells expressing soluble U21NSBPHA, we observe a combination of these two phenotypes; there are intense perinuclear puncta that are never evident in control U373 cells (Fig. 2, c versus a), yet there is slightly more class I MHC labeling of the cell surface in U21NSBPHA cells when compared with U21 cells (Fig. 2, c versus b). We attribute greater surface expression of class I molecules in U21NSBPHA cells to the freedom of soluble U21 from membrane constraint: U21NSBPHA may exist in the ER lumen at lower effective concentration near the membrane, in the vicinity of class I MHC molecules. To confirm that the puncta we observe in U21NSBPHA cells are indicative of lysosomal localization, we performed colocalization experiments with the lysosomal marker lamp2 and found that the puncta colocalize (Fig. 2, compare c and e). Thus, in addition to associating with class I MHC molecules, soluble U21NSBPHA can also divert class I molecules to a lysosomal compartment, albeit less efficiently than membrane-constrained U21.
We also examined the steady state localization of the U21NSBPHA molecules themselves, using an antibody directed against the HA epitope within the SBPHA tag. As with most secreted proteins, at steady state, soluble U21NSBPHA is localized in the secretory pathway, primarily in the ER (Fig. 2d). Strangely, despite its ability to bind to and reroute class I MHC molecules to the lysosomal compartment, soluble U21N molecules localize to a different intracellular compartment than the class I MHC molecules they reroute.
U21 and Class I MHC Molecules Localize Differently
Initially when we expressed full-length U21, we found that class I MHC molecules were redistributed to the lysosomal compartment (Fig. 2) (11). We first hypothesized that U21 escorted class I MHC molecules to a lysosomal compartment, and we fully expected U21 to colocalize with class I molecules in lysosomes. Without an antibody directed against U21, we resorted to tagging U21 at its C terminus with an HA tag (U21HA). Expression of U21HA also resulted in the rerouting of class I MHC molecules to the lysosomal compartment. Interestingly, however, when we examined the localization of the HA-tagged U21 molecules themselves, we found U21HA localized in the ER and secretory pathway (data not shown). Because the U21HA molecule was tagged, we were unable to rule out the possibility that the epitope tag impaired its normal trafficking and localization. To better ascertain the steady state localization of U21, we have now raised a polyclonal antibody to the cytoplasmic tail of U21.
To examine the localization of U21, we expressed nontagged full-length U21 in U373 or HeLa cells using retrovirus-mediated gene transfer and examined U21 and class I MHC localization using immunofluorescence microscopy. We performed double-label immunolocalization experiments with anti-U21 and W6/32. Like the HA-tagged U21, nontagged U21 was localized to the biosynthetic compartments of ER and Golgi (Fig. 3, b and e), whereas class I MHC molecules were located in lysosomes (Fig. 3, a and d).
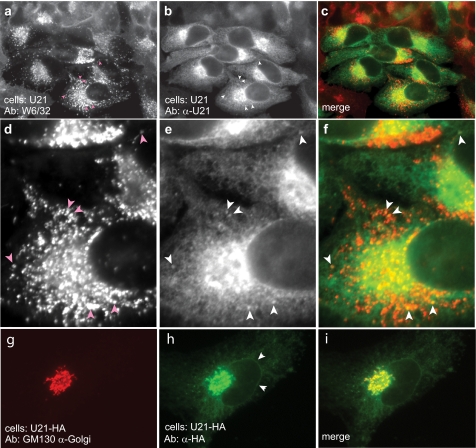
FIGURE 3.
Localization of U21 in HeLa cells. a and b, HeLa cells infected with a U21-encoding puromycin-resistant lentivirus were double-labeled with W6/32 (anti-class I MHC) (a) and anti-U21 (b) prior to selection in puromycin. White arrowheads in b and e denote puncta barely visible in 1–2% of cells labeled with anti-U21. Pink arrows in a and d denote corresponding puncta visible with W6/32. c and f, merged images. d–f, enlarged images of the bottom cell in a–c, with arrowheads. g and h, U373 cells stably expressing HA-tagged U21 were double labeled with GM130, a marker of the cis-Golgi (g), and an anti-HA antibody (12CA5) (h). i, merged image. The white arrowheads in h denote the nuclear envelope, which is contiguous with the ER. Ab, antibody.
Interestingly, a few of the cells labeled with U21 also exhibited faint puncta that colocalize with class I MHC molecules, in addition to the overwhelming ER and Golgi pattern (Fig. 3, b and e, white arrows coincident with pink arrows in a and d). Although visible colocalization of U21 and class I molecules is infrequent and difficult to see, we hypothesize that the steady state abundance of U21 in the biosynthetic pathway “outshines” the U21 that ultimately travels to the lysosomal compartment. One possible explanation for the relative difference in colocalization between U21 and class I molecules is that U21 may be more exquisitely sensitive to lysosomal proteases than class I molecules; thus, U21 may be less visible in the lysosomes at steady state. If this were the case, we would expect that incubation of the cells in lysosomal protease inhibitors might stabilize U21 and class I molecules in lysosomes, and this is indeed the case (10).
To better illustrate the localization of U21 in the Golgi, we performed a double labeling experiment with GM130, a Golgi marker, and HA-tagged U21 (Fig. 3, g–i). U21 in the perinuclear region colocalizes with GM130. ER (and nuclear envelope) localization is also visible (Fig. 3h, arrowheads).
ER-tethered U21 Cannot Reroute Class I Molecules to Lysosomes
The steady state distribution of U21 is puzzling: how can U21 be located primarily in the ER, whereas the class I molecules with which it associates are concentrated in lysosomes? Can U21 somehow modify class I molecules in the ER, rendering them competent to travel to the lysosome without U21 accompaniment? Is the continued association of U21 with class I molecules necessary for diversion of class I molecules to lysosomes?
To determine whether U21 is capable of directing class I molecules to lysosomes while itself remaining in the ER, we placed an ER localization signal on the cytoplasmic tail of U21. There are two types of ER localization signals, both of which signal the retrieval of ER-resident proteins from the cis-Golgi, returning them to the ER (22, 23). A C-terminal cytoplasmic KKXX motif mediates retrieval of transmembrane proteins via cytoplasmic recognition of the ER retrieval signal, and a C-terminal KDEL sequence, found on soluble proteins, is the signal for retrieval of soluble proteins from the cis-Golgi (22, 24–26).
We first engineered a C-terminal KKXX (KKMP) sequence on the cytoplasmic tail of U21. However, the KKMP sequence resulted in the accumulation of U21 at ER exit sites (data not shown). We therefore placed a KDEL ER retrieval signal on the C terminus of the soluble U21NSBPHA. As shown in Fig. 2, soluble U21NSBPHA also diverts class I MHC molecules to the lysosomal compartment, albeit less efficiently than U21 containing an integral membrane domain.
To determine whether our soluble U21NSBPHA-KDEL molecules were successfully localized in the ER at steady state, we performed pulse-chase experiments with U21NSBPHA-KDEL cells. At each chase point, we recovered class I molecules with W6/32 and digested half of the immunoprecipitates with endoglycosidase H (EndoH), which cleaves N-linked glycans, but only if they have not progressed beyond the early Golgi. When a glycoprotein travels beyond the medial Golgi, it becomes resistant to EndoH digestion. Thus, assessment of EndoH sensitivity is a way to monitor the progress of a glycoprotein as it traverses the secretory pathway. Immunoprecipitation of class I molecules with W6/32 recovers U21NSBPHA-KDEL molecules (Fig. 4a, compare lanes 4–6 with lanes 1–3), demonstrating that the truncated KDEL-fused U21NSBPHA molecules associate with class I MHC molecules.
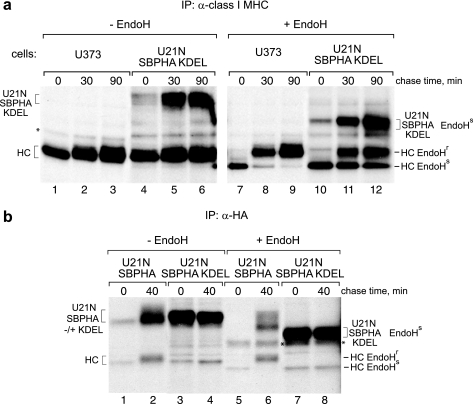
FIGURE 4.
U21NSBPHA-KDEL retains class I molecules in the ER. a, U373 or U21NSBPHA-KDEL cells were pulsed with [35S]methionine for 10 min and chased for the indicated times. Class I molecules were recovered with W6/32, digested with EndoH, and resolved by SDS-PAGE. The EndoH-sensitive and -resistant forms of the class I heavy chain (HC) and U21NSBPHA-KDEL are indicated. b, HA-tagged U21 was recovered from U21NSBPHA and U21NSBPHA-KDEL cells digested with EndoH and resolved by SDS-PAGE. The asterisks denote a nonspecific contaminating polypeptide.
In U21NSBPHA-KDEL cells, approximately half of the class I molecules remain EndoH-sensitive at 30 min (compare acquisition of EndoH resistance; Fig. 4a, lanes 10–12 versus lanes 7–9). If U21NSBPHA-KDEL were expressed in every cell at levels comparable with the expression levels of class I MHC molecules, we would expect all of the class I MHC molecules to remain EndoH-sensitive. The U21NSBPHA-KDEL cells, however, are pooled neomycin-resistant cells expressing heterogeneous levels of U21NSBPHA-KDEL molecules. The EndoH-resistant class I molecules present in lanes 11 and 12 likely represent class I molecules from cells with low levels of U21NSBPHA-KDEL expression.
All of the ER-tethered U21NSBPHA-KDEL molecules that coprecipitate with the anti-class I MHC antibody are EndoH-sensitive (Fig. 4a, compare lanes 10–12 with lanes 4–6). Likewise, all of the class I molecules associated with the KDEL-retained version of U21NSBPHA are EndoH-sensitive (Fig. 4b, compare class I heavy chain molecules in lanes 7 and 8 with lanes 5 and 6). Thus, the KDEL sequence successfully results in the steady state localization of U21N molecules in the ER; when U21 is localized in the ER, the class I MHC molecules with which it associates are also retained in the ER, suggesting that to divert class I MHC molecules, U21 must accompany them out of the ER and travel with them through the Golgi.
To examine the localization of class I molecules in U21NSBPHA-KDEL cells, we performed double-label immunofluorescence localization experiments, using W6/32 to examine the localization of class I MHC molecules and an antibody directed against the ER-resident protein, protein-disulfide isomerase, to mark the ER. Control U373 cells express class I MHC molecules on their cell surface, and protein-disulfide isomerase is distributed in the reticular pattern characteristic of the ER (Fig. 5, a and b). For comparison, in cells expressing U21, class I MHC molecules are localized to lysosomes (Fig. 5c). Class I MHC molecules in cells expressing soluble U21NSBPHA are present in lysosomes and on the cell surface (Fig. 5e). However, when U21NSBPHA molecules are retained in the ER with a KDEL sequence, class I MHC molecules also remain in the ER (Fig. 5, g and h), corroborating the pulse-chase experiments shown in Fig. 4 and suggesting that W6/32-reactive class I molecules are bound by U21 in the ER and that U21 must be able to leave the ER with class I MHC molecules to effect their diversion to lysosomes.
FIGURE 5.
Class I MHC molecules in U21-KDEL cells are retained in the ER. Top panels, control U373 cells, U21-, U21NSBPHA-, and U21NSBPHA-KDEL-expressing cells were immunolabeled with W6/32 to examine class I localization. Bottom panels, for comparison, these cells were labeled with anti-protein-disulfide isomerase (PDI) as a marker for ER immunolabeling. Ab, antibody.
Is U21 Retained in the ER?
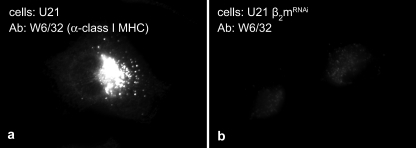
The observation that the majority of U21 is present in the ER at steady state (Fig. 3b) prompted us to delve more deeply into the trafficking of U21: is U21 retained in the ER until it binds to a class I MHC molecule, or is it synthesized and immediately routed to lysosomes, regardless of whether it is bound to class I? To address this question, we examined U21 trafficking in the absence of class I molecules. Because most nucleated cells express both alleles of three different class I MHC genes (HLA-A, -B, and -C), rather than knock down all six of the class I heavy chains, we employed retrovirally encoded RNAi to knock down the class I MHC light chain, β2m. In the absence of β2m, class I heavy chain molecules cannot fold properly, and any remaining unfolded class I molecules are unable to bind to U21 (18). The resulting reduction in W6/32-reactive class I MHC molecules is illustrated in Fig. 6, where W6/32 labeling of U21-expressing cells (Fig. 6a) is depicted at the same exposure as U21 cells lacking β2m (Fig. 6b). Immunoblots confirmed knockdown of β2m to undetectable levels (21).
FIGURE 6.
Cells deficient in β2m lack W6/32 reactivity. Immunofluorescent detection of class I MHC molecules in U21-expressing (a) or U21 + shβ2m (b) U373 astrocytoma cells with the conformation-specific W6/32 antibody.
If U21 must first associate with class I molecules to leave the ER, we reasoned that U21 might accumulate in the ER when folded class I MHC molecules are unavailable. To compare U21 trafficking in cells lacking β2m, we performed pulse-chase and EndoH analysis of U21 in cells expressing U21 and lacking β2m (Fig. 7). At each chase point, we recovered U21 and class I molecules with either an antibody directed against U21 (Fig. 7a) or W6/32 (Fig. 7b), and half of the immunoprecipitates were digested with EndoH.
FIGURE 7.
EndoH sensitivity of U21 is unchanged when folded class I molecules are unavailable. U21-expressing or U21 + shβ2m (β2m kd) U373 astrocytoma cells were pulsed with [35S]methionine for 15 min and chased for the indicated times. Immunoprecipitates (IP) with either anti-U21 (a) or W6/32 (b) were divided, and half of the recovered material was treated with endoglycosidase H, as noted. U21 and class I heavy chains (HC) are noted, as are migration of EndoH-sensitive and EndoH-resistant forms of U21. Of note, EndoH-sensitive U21, which loses four N-linked glycans, migrates slightly more slowly than EndoH-sensitive class I MHC, so that the two bands are indistinguishable from one another (lanes 4). The double asterisk in b denotes a possible contaminating cross-reactive polypeptide (visible in lanes 5, 7, 8, 10, and 11). c, U21-expressing U373 or K562 cells were pulsed and chased and treated with EndoH as described above. U21, class I MHC heavy chains, and their EndoH-sensitive and -resistant counterparts are noted.
As expected, in the absence of β2m, class I molecules cannot be recovered in immunoprecipitation with the conformation-specific anti-class I MHC antibody W6/32 (Fig. 7b, compare lanes 1–3 with lanes 7–9), nor can they be coprecipitated with U21 molecules (Fig. 7a, compare lanes 1–3 with lanes 7–9) (18). Of note, however, we must recover enough nonlabeled folded class I molecules with W6/32 to coprecipitate some labeled U21 (Fig. 7b, lanes 8, 9, 11, and 12).
In both U21 cells and U21 cells lacking β2m, U21 molecules become EndoH-resistant at the later chase points (Fig. 7a, lanes 5, 6, 11, and 12). After 120 min, the amount of U21 remaining EndoH-sensitive was reduced by about 3-fold, both in U21 cells and in U21 cells lacking β2m. Thus, U21 appears to have a similar half-life and to leave the ER with similar kinetics, regardless of whether folded class I MHC molecules are present, suggesting that the trafficking of U21 is not dependent upon its association with class I molecules. Fig. 7b shows the reciprocal immunoprecipitation with W6/32. Note that some folded class I MHC molecules remain in the cells with knocked down β2m, because a small amount of labeled U21 is coimmunoprecipitated with nonlabeled class I MHC molecules (Fig. 7b, lanes 7–12).
As an alternative approach to β2m depletion, we also performed a pulse-chase experiment recovering U21 from U21-expressing K562 cells. K562 cells are a myeloid-derived cell line lacking class I MHC molecules. For comparison, we recovered U21 from U21-expressing U373 cells. As previously shown, when we recover U21 from U21-expressing U373 cells, class I heavy chain molecules coimmunoprecipitate (Fig. 7c, lanes 1–4). From U21-expressing K562 cells, although comparable amounts of U21 are recovered, no coprecipitating class I heavy chain molecules are observed (Fig. 7c, lanes 5–8). Just as in the U373-U21 cells lacking β2m, U21 molecules in K562 cells become EndoH-resistant at the later chase points (Fig. 7c, compare lanes 13–16 with lanes 9–12). Thus, in K562 cells as well, U21 appears to have a similar half-life and leave the ER with similar kinetics, regardless of whether class I MHC molecules are present, suggesting that the trafficking of U21 is not dependent upon its association with class I molecules.
Does U21 Modify the Cytoplasmic Tail of Class I Molecules?
Because lysosomal trafficking generally involves a cytoplasmic lysosomal sorting signal, we initially hypothesized that U21 might contain a sorting signal within its cytoplasmic tail. We have shown that the cytoplasmic tail of U21 is not necessary for the ability of U21 to redirect class I MHC molecules to the lysosomal compartment (18) (Fig. 2, c and e). Because the cytoplasmic tail of U21 proved unnecessary, we next asked whether the lumenal domain of U21, through association with the lumenal portion of class I MHC molecules, might induce a conformational change that is transduced through the lipid bilayer, such that the cytoplasmic tail of the class I heavy chain could then contain or receive a signal to traffic to lysosomes. There is some suggestion of precedent for this phenomenon: in 1995, Little et al. (27) raised an antibody directed against the cytoplasmic tail of class I heavy chain molecules. They were surprised to find that the antibody recognized class I heavy chains only when the lumenal portion of the molecule was unfolded, suggesting that the conformation of the lumenal domain of the class I molecule influences the conformation of its cytoplasmic tail.
We therefore hypothesized that if U21 binding renders the cytoplasmic tail of the class I heavy chain molecule able to contain or receive a signal for trafficking to lysosomes, then U21 should not be able to reroute tailless class I molecules to lysosomes. To test this hypothesis, we replaced the cytoplasmic tail of the class I HLA-A2 heavy chain molecule with an HA epitope tag and examined the subcellular localization of this tailless HLA-A2 molecule in cells that expressed U21 (see Fig. 8a).
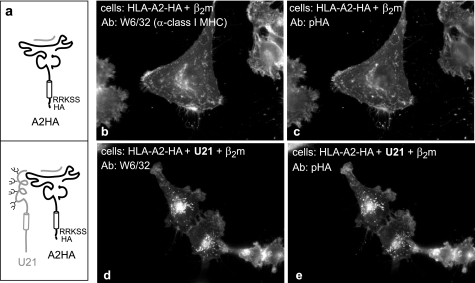
FIGURE 8.
Tailless HLA-A2 molecules are rerouted by U21. a, cartoon depicting the HLA-A2-TMHA fused molecules expressed in U373 cells in the absence or presence of U21. The HLA-A2 molecules consist of the transmembrane region of HLA-A2 and the membrane-proximal RRKSS sequence, followed by an HA tag. b–e, immunofluorescent detection of endogenous and HA-tagged class I MHC molecules with W6/32 (b and d) and an anti-HA polyclonal antibody (HA.11) (c and e). The cells and antibodies are noted. Ab, antibody.
Newly synthesized class I MHC heavy chains require associated β2m light chains to fold properly. We have found that when we overexpress class I MHC molecules in cells, the endogenous β2m appears limiting: often, class I molecules are inappropriately localized in the ER, where they likely remain unfolded. When we simultaneously infect the cells with a retrovirus encoding β2m, the overexpressed HLA molecules are able to traffic to the cell surface (data not shown). For this reason, we include β2m with every transduction of HLA-A2 molecules.
The HA-tagged HLA-A2-HA molecule behaved similarly to full-length HLA molecules. In control U373 cells expressing the tailless HLA-A2-HA molecule, the labeling pattern with W6/32 and anti-HA antibodies is virtually indistinguishable (Fig. 8, b and c). Likewise, when U21 is expressed in these cells, the labeling for the tailless HLA-A2-HA molecules was almost indistinguishable from that of HLA-A, -B, and -C with W6/32 (Fig. 8, d and e).
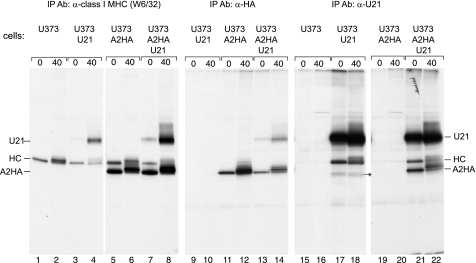
The tailless HLA-A2-HA molecules behaved indistinguishably from endogenous class I MHC molecules in immunoprecipitation experiments as well. In immunoprecipitations with W6/32, we recovered both endogenous class I heavy chains as well as the more quickly migrating tailless A2-HA molecules (Fig. 9, lanes 1–8). The identity of the tailless A2-HA was confirmed in immunoprecipitations with an anti-HA antibody (Fig. 9, lanes 9–14), and as suggested by the immunofluorescence pattern in Fig. 8 (b and d), immunoprecipitation of HA-tagged A2 molecules resulted in the recovery of U21 molecules (Fig. 9, lanes 13 and 14). Likewise, when U21 molecules were immunoprecipitated from cells expressing the tailless A2-HA molecules, both endogenous HLA as well as HLA-A2-HA molecules were recovered (Fig. 9, lanes 15–22). Thus, neither the cytoplasmic tail of U21 nor the cytoplasmic tail of class I MHC heavy chain molecules appears to be necessary for U21-mediated diversion of class I MHC molecules to the lysosomal compartment.
FIGURE 9.
Tailless HLA-A2 molecules associate with U21. U373 or U373 cells expressing U21, A2HA, or A2HA and U21 were pulsed with [35S]methionine for 15 min and chased for 0 and 40 min, as noted. Class I molecules were recovered with W6/32 or 12CA5 (anti-HA). U21 molecules were recovered with MCW50. The migration positions of U21, class I heavy chain molecules (HC), and the chimeric tailless HLA-A2HA molecules are indicated. The asterisk (lanes 17 and 18) denotes a possible breakdown product present only in the U21 cells. Ab, antibody; IP, immunoprecipitation.
Our tail-deleted HA-tagged HLA-A2 molecule consists of an HA tag fused to the membrane-proximal RRKSS sequence of the cytoplasmic tail (Fig. 8a). These 5 amino acids were retained to allow the class I heavy chain to properly insert into the membrane (28). Recently, this membrane-proximal lysine (in bold type) in the tail of class I heavy chain molecules was found to be targeted by the Kaposi's sarcoma-associated herpesvirus (KSHV) K5 ubiquitin ligase (29). Ubiquitination of this lysine residue was shown to be important for endocytosis of HLA-A2 molecules. We therefore hypothesized that U21 association with the class I heavy chain molecule might change the conformation of the cytoplasmic tail in such a way as to make this membrane-proximal lysine available for ubiquitination by a cellular ubiquitin ligase. The cellular MARCH (membrane-associated RING-CH) ubiquitin ligases, for example, discovered by virtue of their homology to the KSHV K3 and K5 ubiquitin ligases, would be excellent candidates for such a cellular partner for U21 (30).
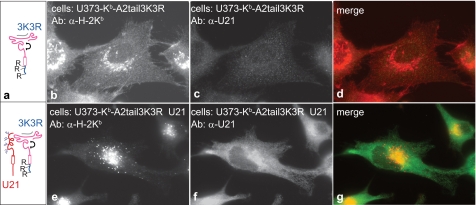
To explore the possibility that U21-mediated trafficking of class I molecules to lysosomes depends upon the presence of lysines in the cytoplasmic tail of the class I MHC molecule, we mutated the three lysines in the cytoplasmic tail of HLA-A2 (3K→3R). To distinguish the mutant class I molecules from endogenous HLA molecules, we fused the mutant 3K→3R tail of HLA-A2 to the cytoplasmic and transmembrane regions of murine H-2Kb (Fig. 10a). We have shown that murine H-2Kb is efficiently diverted to lysosomes by U21 (31).
FIGURE 10.
U21 reroutes H-2Kb-HLA-A2-tail 3K3R chimeric molecules. a, cartoon depicting the H-2Kb-HLA-A2-tail 3K→3R chimeric molecules expressed in U373 cells in the absence or presence of U21. b–g, U373 cells expressing H-2Kb-A2tail-3K3R without (b–d) or with (e–g) U21 were labeled with Y3 anti-H-2Kb tail mAb and the anti-U21 polyclonal antibody MCW50, as indicated. The merged images are shown in d and g. Ab, antibody.
When H-2Kb molecules fused to the lysine-less HLA-A2 3K→3R cytoplasmic tail are expressed in U373 cells, they localize to the plasma membrane (Fig. 10b). When U21 is introduced into these cells, the chimeric Kb-A2 3K→3R molecules are relocalized to the lysosomal compartment (Fig. 10e). Double labeling of these cells with our anti-U21 Ab demonstrates U21 expression (Fig. 10, c and f). Thus, mutation of the lysines in the cytoplasmic tail of HLA-A2 did not impair the ability of U21 to reroute chimeric murine-human class I molecules, and we may therefore rule out ubiquitin conjugation of the cytoplasmic tail as a requirement for U21-mediated trafficking of class I MHC molecules to lysosomes.
Is Glycosylation of U21 Important?
U21 from HHV-7 contains four N-linked glycans. N-Linked glycans have been demonstrated to be important for the intracellular trafficking of several integral membrane proteins (32–34). Because the cytoplasmic tails of both U21 and class I MHC molecules are not responsible for diversion of class I molecules to the lysosomal compartment, we hypothesize that the lumenal domain of U21 must contain a signal or sequence that is recognized by an unknown cellular protein (protein X) that mediates sorting. One possibility is that this cellular protein has affinity (at least in part) for the N-linked glycan moieties in the lumenal portion of U21.
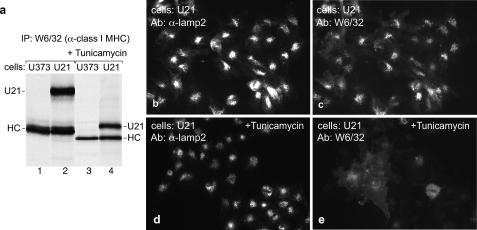
To determine whether the N-linked glycans are important for the ability of U21 to divert class I molecules to lysosomes, we incubated U21 cells in the presence of tunicamycin, a cell-permeable inhibitor of N-linked glycosylation. Because glycosylation is involved in proper protein folding, we were initially concerned with whether, in the presence of tunicamycin, U21 and class I molecules would achieve their proper conformation. U21 associates exclusively with properly folded class I molecules (18). Therefore, if U21 and class I molecules can associate in the presence of tunicamycin, then they must be folded properly. Indeed, in tunicamycin-treated cells, nonglycosylated U21 molecules are recovered in immunoprecipitations with W6/32, suggesting that tunicamycin does not affect the folding of class I molecules (Fig. 11a, compare lanes 3 and 4 with lanes 1 and 2).
FIGURE 11.
Tunicamycin interferes with U21-mediated diversion of class I molecules to lysosomes. a, U373 cells or U373 cells expressing U21 were labeled with [35S]methionine for 15 min in the absence or presence of tunicamycin, as noted. Class I MHC molecules were recovered with W6/32. The glycosylated and nonglycosylated class I heavy chain (HC) and U21 molecules are noted. b–e, low magnification U21 cells immunolabeled with anti-lamp2 (b and d) or class I MHC (W6/32) (c and e) after 6 h in the absence (b and c) or presence (d and e) of tunicamycin. Ab, antibody; IP, immunoprecipitation.
We next examined class I MHC localization in U21 cells after incubation for 6 h in tunicamycin. In tunicamycin-treated cells, class I MHC molecules no longer appeared intense, punctate and perinuclear, and more labeling was apparent at the cell surface (Fig. 11, compare c and e), suggesting that glycosylation of U21 is important for diversion of class I molecules to lysosomes. The caveat to this experiment, although it is suggestive, is that tunicamycin prevents glycosylation not only of U21 and class I MHC molecules but also of every other glycoprotein in the cell. In addition to possible mediation of intracellular trafficking, glycosylation is also important for proper protein folding. Of note, however, localization of the lysosomal membrane protein lamp2 is not affected by tunicamycin treatment (Fig. 11, b and d), suggesting that tunicamycin treatment does not prohibit sorting of all membrane proteins to lysosomes.
Still, we could not be sure that tunicamycin does not affect proper folding of other cellular proteins necessary for U21-mediated diversion of class I MHC molecules to lysosomes. Thus, these tunicamycin experiments are only suggestive of a role for N-glycosylation in U21-mediated diversion of class I MHC molecules to lysosomes. The better experiment to address the role of glycosylation in U21 function is to mutate the four N-linked glycosylation consensus sites in U21, preventing glycosylation of U21 alone.
In constructing U21 molecules with mutated N-glycosylation sites, the possibility existed that mutation of a particular amino acid(s) would detrimentally affect overall folding of the U21 molecule. Because glycosylation is not necessary for the association of U21 with class I MHC molecules (Fig. 11), we reasoned that, as an indicator of proper conformation, mutation of the N-glycosylation sites in U21 should result in a U21 molecule that retains its ability to associate with class I molecules. However, when we mutated the four N-linked glycosylation sites in HHV7 U21 (4N→QSBPHA) and expressed the mutant molecule in U373 cells, we could not coimmunoprecipitate class I MHC molecules with the U21 4N→QSBPHA mutant (data not shown), suggesting the possibility that mutation of the asparagines in some way disrupted proper conformation of the U21 molecules.
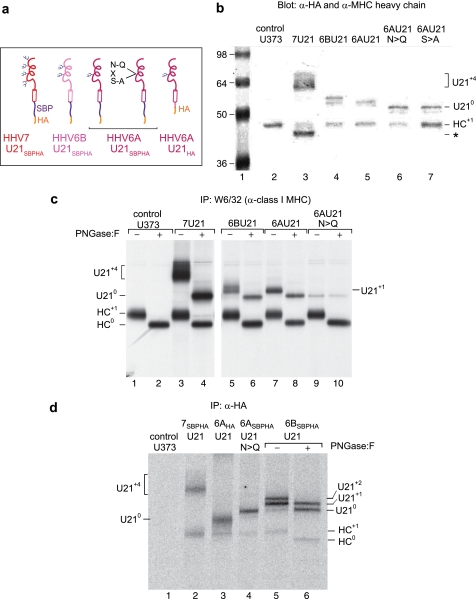
Fortuitously, HHV-6A U21, which is ∼30% identical to HHV-7 U21 at the amino acid level and also functions to reroute class I MHC molecules to lysosomes (10), conserves only one of the four N-linked glycosylation sites (Fig. 13a). HHV-6B U21, which is ∼90% identical to HHV6A U21, conserves two of the four N-linked glycosylation sites of HHV-7 U21 (10). Thus, rather than engineer mutations at all four sites in HHV-7 U21, we mutated the single conserved NXS glycosylation site in HHV-6A U21SBPHA at position 164 to glutamine (N164Q). In addition, we generated a separate mutation of serine 166 to alanine (S166A) (data not shown). For these experiments, we used the SBPHA-tagged form of HHV-6A U21, so that we could detect expression and localization of the mutant U21 molecules using an anti-HA antibody. This HHV-6A U21SBPHA molecule contains the U21 lumenal and transmembrane domain, but the cytoplasmic tail is replaced by the SBPHA tag. We have previously shown that the SBPHA-tailed molecules function similarly to the nontagged version in their ability to reroute class I MHC molecules to lysosomes (10).
FIGURE 13.
The HHV6A U21 glycosylation site mutants are expressed and can associate with class I MHC molecules. a, cartoon depicting the different U21 chimeric molecules expressed to assess the potential role of N-linked glycans. U21 molecules are as noted, in red (HHV7), pink (HHV6B), and magenta (HHV6A). The U21HA molecule consists of full-length HHV6A U21 with an appended C-terminal HA tag. All other molecules have their cytoplasmic tails replaced with an SBPHA tag, as depicted. b, immunoblot of cells expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants. All of the cells express the chimeric U21SBPHA versions of U21, but they are not noted in the figure because of space constraints. The membrane was probed with anti-HA and anti-class I MHC heavy chain (HC10). The migration position of HHV-7 U21 with four N-linked glycans (U21+4) is noted, as is the position of nonglycosylated U21 (U210) and the singly glycosylated class I MHC heavy chain (HC+1). The asterisk denotes a likely proteolytic fragment of HHV-7 U21SBPHA that is reactive with the HA.11 Ab (lane 3). The molecular weight standards are shown in lane 1. c, immunoprecipitation (IP) of class I MHC molecules from [35S]methionine-labeled cells (40-min pulse) expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants. All of the cells express the chimeric U21SBPHA versions of U21, but they are not noted in the figure because of space constraints. The migration position of HHV-7 U21 with four N-linked glycans (U21+4) is noted, as is the position of nonglycosylated U21 (U210) and the singly glycosylated class I MHC heavy chain (HC+1). Class I MHC molecules were recovered from cells with W6/32, and half of the immunoprecipitates were digested with PNGase:F and resolved by SDS-PAGE. The migration position of deglycosylated class I heavy chains (HC0) and U21 (U210) is indicated. d, immunoprecipitation of HA-tagged U21 from [35S]methionine-labeled cells (40-min pulse) expressing HHV-7, HHV6B, and HHV6A U21 molecules, as well as HHV6A U21 glycosylation mutants and HHV6AHA molecules. For HHV6B U21SBPHA cells, half of the immunoprecipitates were subject to PNGase treatment, as described above (lanes 5 and 6). The migration positions of U21 and class I heavy chains are noted, as in b and c.
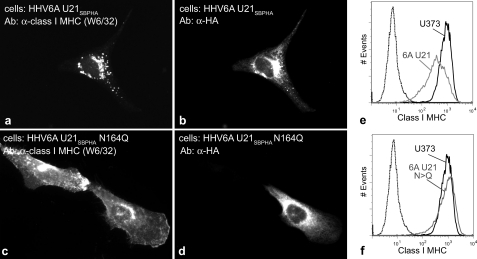
We used retrovirus-mediated gene transfer to stably express these molecules in U373 astrocytoma cells. When we expressed the N164Q mutant of HHV-6A U21, the localization of class I MHC molecules did not change: class I molecules were localized on the cell surface (Fig. 12, compare c with a, where d shows adjacent U21SBPHA-expressing and nonexpressing cells). We obtained identical results with the S166A mutant (data not shown). To analyze the entire population of these cells as a whole, we performed flow cytometric analysis of surface class I expression (Fig. 12, e and f), where the black trace shows class I MHC levels on the surface of control U373 cells and the gray trace shows surface down-regulation of class I MHC molecules in cells expressing either HHV-6A U21SBPHA (Fig. 12e) or the HHV-6A U21SBPHA N164Q mutant (Fig. 12f).
FIGURE 12.
Mutation of the N-linked glycosylation consensus site in HHV6-U21 affects U21-mediated rerouting of class I molecules to lysosomes. a–d, double-label immunolocalization of class I MHC molecules using W6/32 in cells expressing HHV-6A U21 or HHV6A U21 with its sole N-linked glycosylation site mutated to glutamine (N164Q), as noted. The SBPHA-tagged HHV6 U21 molecules are labeled with an anti-HA Ab (HA.11). e and f, flow cytometric analysis to detect surface class I MHC molecules in U373 cells (black traces) and U373 cells expressing HHV6 U21 or HHV6 U21 Asn → Gln (gray traces), as noted. The dotted traces in e and f represent cells incubated in the absence of phosphatidylethanolamine-conjugated anti-HLA-A, -B, or -C. Ab, antibody.
To ensure that each of these cell populations expressed similar levels of the U21SBPHA and U21SBPHA N164Q molecules, we performed immunoblotting with anti-HA and anti-class I heavy chain antibodies (Fig. 13b). Lane 2 shows class I heavy chains from control cells. For comparison, HHV7 and HHV6B U21SBPHA are shown in lanes 3 and 4. HHV7 U21SBPHA is expressed at somewhat higher levels and migrates more slowly, reflecting its four N-linked glycans (Fig. 13, b, lane 3, and a). HHV6B U21SBPHA is heterogeneously glycosylated with either one or two glycans (Fig. 13b, lane 4), and HHV6A U21SBPHA migrates at a position identical to HHV6B U21SBPHA with one N-glycan (lane 5). Both the N164Q and S166A mutants are expressed and migrate at a position commensurate with the predicted size for nonglycosylated U21 (Fig. 13b, lanes 6 and 7). The four HHV6 U21SBPHA proteins are expressed at similar levels in these stable cell lines, corresponding with the HA expression we observed in our microscopic evaluation of the cells (10).
We were concerned that the N164Q mutation might interfere with proper folding of the U21 molecule, rendering the mutated molecule unable to associate with class I molecules. To examine whether U21 N164Q could associate with class I molecules, we performed coimmunoprecipitation experiments with W6/32 to recover class I and associated U21 N164Q, as well as the reciprocal immunoprecipitation with an anti-HA antibody to recover U21 N164Q and associated class I MHC molecules. We recovered properly folded class I MHC molecules using the W6/32 antibody and treated half of the immunoprecipitates with PNGase:F to demonstrate the mobility shift in the molecules in the absence of their N-linked glycans. From control U373 cells, we immunoprecipitate the class I heavy chain at ∼43 kDa and observe a ∼2.5-kDa shift after PNGase:F treatment, corresponding to the single N-linked glycan on the class I heavy chain (Fig. 13c, compare lanes 1 and 2). From U373 cells expressing HHV7 U21SBPHA, we recovered class I molecules as well as coprecipitating U21SBPHA. The shift in mobility after PNGase:F treatment reflects the four N-linked glycans on HHV7 U21 (Fig. 13c, lanes 3 and 4). From cells expressing HHV6B and HHV6A U21SBPHA, the pattern is similar, although HHV-6B U21SBPHA is heterogeneously glycosylated on its two N-linked glycosylation sites, leading to its migration as a doublet; it migrates as a single ∼53-kDa polypeptide after PNGase:F treatment (Fig. 13c, lanes 5 and 6). We have described the heterogeneously glycosylated nature of HHV6B U21 in a previous study (10). HHV6A U21 contains only a single N-linked glycan, and when the asparagine is mutated, recovery of class I MHC molecules with W6/32 coprecipitates a polypeptide that migrates at precisely the same mobility as the deglycosylated HHV6A U21SBPHA (Fig. 13c, lanes 8 and 9). Predictably, PNGase:F treatment does not result in a mobility shift of the mutant U21 N164Q molecule, whereas class I heavy chain molecules shift appropriately (Fig. 13c, lane 10). These results suggest that the N164Q mutant is able to associate with class I MHC molecules. Of note, however, we recovered less labeled U21SBPHA N164Q from U21SBPHA N164Q cells than from cells expressing the nonmutant form of U21SBPHA (Fig. 13c, compare lanes 5, 7, and 9), although all three of these cell lines express equal steady state levels of each protein (Fig. 13b, lanes 4–6). It is possible that the Asn → Gln mutation affects the trafficking of the U21 protein through the secretory pathway such that the percentage of pulse-labeled U21 associated with pulse-labeled class I molecules is different in these cells. It is also possible that the Asn → Gln mutant may diminish the ability of U21 to associate with class I MHC molecules.
We also performed reciprocal immunoprecipitations, recovering the U21SBPHA molecules with an anti-HA antibody. As expected, from control U373 cells we recover nothing with the anti-HA antibody (Fig. 13c, lane 1). Recovery of HHV7 U21SBPHA results in coprecipitation of class I MHC molecules (Fig. 13d, lane 2). As a control to demonstrate the similarity between full-length U21 and the U21SBPHA molecules, we included immunoprecipitations from cells expressing the HHV6A U21-HA molecule, which contains an HA tag at the end of its cytoplasmic tail (Fig. 13a). This HHV6A U21HA molecule migrates faster than HHV6A U21SBPHA, because the SBPHA tag is larger than the cytoplasmic tail (Fig. 13d, lane 3). Of note, the HHV6A U21HA, HHV6A U21SBPHA N164Q, and the HHV6B U21SBPHA all coprecipitate a similar amount of class I MHC heavy chain molecules (Fig. 13d, lanes 3–5), suggesting that they are similarly capable of association with class I MHC molecules. An incompletely digested HHV6B U21SBPHA is shown for comparison, to illustrate that the migration position of the deglycosylated U21 corresponds to the migration position of the N164Q mutant (Fig. 13, d, lane 6, and c, lanes 6, 8, and 9). These experiments thus suggest that the proper glycosylation of U21 is necessary for its ability to reroute class I MHC molecules to the lysosomal compartment but not for its association with class I MHC molecules themselves.
DISCUSSION
The mystery of how this viral integral membrane protein can reroute class I MHC molecules to the lysosomal compartment has yet to be solved, but the experiments described herein bring us closer to an understanding of its mechanism. Here we show that U21 can reroute class I MHC molecules to the lysosomal pathway even as a soluble protein, without being constrained within the membrane by its transmembrane domain.
We show for the first time the immunolocalization of U21. We were surprised to find that, rather than localize in the lysosomal compartment, U21 is largely localized in the ER and biosynthetic compartment. This is in contrast to the gp48 immunoevasin from murine cytomegalovirus (MCMV); like U21, gp48 binds to class I MHC molecules and diverts them to lysosomes. Unlike U21, gp48 colocalizes in lysosomes with the class I MHC molecules it reroutes (14). Steady state distribution of U21 in the ER prompted the question of whether U21 could have its effects upon class I molecules without ever leaving the ER.
Using a fused KDEL ER retrieval signal, we demonstrated that when U21 is prevented from leaving the ER, class I molecules colocalize with U21 in the ER; thus, U21 and class I must leave the ER together en route to the lysosomal compartment. Thus, the normal steady state localization of U21 in the ER does not reflect its movement within the cell as it works to reroute class I MHC molecules. It is possible, for example, that our anti-U21 Ab can recognize U21 while it is in the ER but cannot easily recognize U21 that is en route to lysosomes, perhaps because of its association with a cellular protein that effects its trafficking to the lysosomal compartment. Alternatively, steady state localization of U21 in the ER may result from U21 being actively retained in the ER until association with a class I molecule renders it competent to leave. To examine this possibility, we asked whether the trafficking of U21 was altered in the absence of properly folded class I MHC molecules. We found that U21 leaves the ER normally, regardless of the presence of class I molecules.
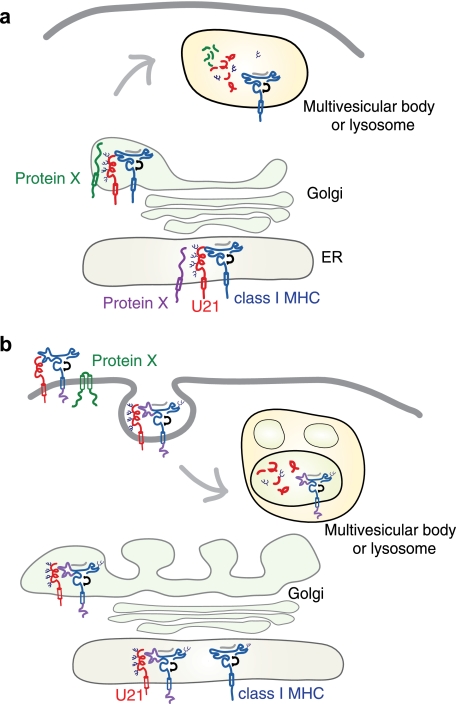
The process of trafficking of integral membrane proteins to the lysosomal pathway is generally mediated by proteins that recognize various cytoplasmic sorting signals. The lamp proteins, for example, which comprise perhaps as much as 70% of the total lysosomal membrane protein content, contain tyrosine-based sorting signals in their cytoplasmic tails that are recognized by the clathrin adaptor proteins AP-1 and AP-3. These adaptor protein complexes mediate sorting to the endolysosomal compartment. In constructing a model for U21-mediated trafficking of class I MHC molecules to lysosomes, the simplest is one exemplified by the MCMV gp48 immunoevasin (14). MCMV gp48 contains a di-leucine sorting signal in its cytoplasmic tail that requires functional AP-1 and AP-3 clathrin adaptor complexes. These adaptor complexes mediate sorting of the gp48-class I MHC complex to the lysosomal compartment (35). We initially postulated a similar mechanism for U21, but instead, we found that U21 can affect the trafficking of class I molecules even when its cytoplasmic tail is deleted (11) and even when the U21 molecule is in a soluble secreted form (Fig. 2). Thus, if the lysosomal sorting signal utilized by U21 is cytoplasmic, we can envision two possible models: either the sorting signal must exist on the class I molecule or a third, cellular protein(s) associates with U21 and class I molecules that contains the information necessary to reroute the class I MHC-U21 complexes (Fig. 14a).
FIGURE 14.
Models depicting U21-mediated diversion of class I MHC molecules to the lysosomal compartment. U21 (red) associates with and diverts class I MHC molecules (blue) to a lamp1- and lamp2-positive lysosomal compartment. In a, U21-class I MHC complex associates with a cellular protein, Protein X (green or purple), which contains lysosomal targeting information. The association with Protein X could occur in the ER (purple) or later on in the biosynthetic pathway (green), accounting for a short-lived association with U21, and the lack of apparent coimmunoprecipitation of U21 with Protein X. Trafficking to lysosomes may involve a direct route from the Golgi to the lysosomal compartment or may involve arrival at the plasma membrane and subsequent routing through endosomes (depicted in b). In b, U21 (red) associates with class I MHC molecules and causes a conformational change in the class I molecule that is transduced through the membrane to the cytoplasmic tail. Through its lumenal association with U21, the cytoplasmic tail of the class I MHC molecule is somehow rendered competent to be modified by a Protein X (green), which then targets the complex of U21 and class I to an endolysosomal compartment.
To address the first of these possibilities, we examined whether the cytoplasmic tail of class I molecules might be involved in their targeting to lysosomes. Class I MHC molecules do not travel to the lysosomal compartment by default; they have a relatively long half-life and reside primarily at the cell surface. In some instances, however, their trafficking to a degradative compartment is warranted. If, for example, a class I MHC molecule were to lose its peptide upon reaching the extracellular milieu, it might lose its conformation and become nonfunctional. In such a situation, a means of internalization, trafficking through the endolysosomal pathway, and subsequent degradation has been proposed: ubiquitination of the cytoplasmic tail of class I MHC molecules by proteins of the MARCH family of ubiquitin ligases has been proposed as a possible mechanism for removing unfolded class I MHC molecules from the cell surface (30). To examine whether the cytoplasmic tail of class I molecules might be involved in their targeting to lysosomes by U21, we replaced the cytoplasmic tail of HLA-A2 with an HA tag and found U21 to be just as effective with the HA-tagged tailless class I molecules as with the other class I molecules in the cell. Thus, the hypothesis that U21 binds to class I molecules rendering the cytoplasmic tail amenable to modifications that would target the U21-class I complex to lysosomes is likely incorrect (Fig. 14b).
Because expression of a single viral protein results in the rerouting of class I molecules to lysosomes, we hypothesize that rather than encode a novel mechanism for trafficking to lysosomes, U21 likely acts to usurp a normal cellular means of lysosomal sorting. Neither the cytoplasmic tail of the class I MHC molecule nor the cytoplasmic tail of U21 are necessary for U21-mediated trafficking of class I molecules to the lysosome; thus we are left to assume that U21 must associate in the lumen with a third, cellular protein. This cellular protein (protein X), which likely contains lysosomal targeting information in its cytoplasmic tail, might bind to U21 soon after the molecules are synthesized, or it may mediate the lysosomal sorting event later in the secretory pathway, associating with U21 and class I in the trans-Golgi network or even later, at the plasma membrane (Fig. 14a). If our model is correct, and U21 associates with both class I molecules and this cellular protein X, then the association must occur within the lumen, because neither cytoplasmic tail is necessary for the rerouting of class I molecules to lysosomes.
One particularly attractive candidate for protein X is the mannose 6-phosphate receptor (M6PR). Sorting of soluble lysosomal hydrolases is mediated by the M6PR binding to mannose 6-phosphate residues on N-linked sugar moieties of these hydrolases. Although U21 is an integral membrane protein, it does contain N-linked glycans, which could be mannose 6-phosphorylated and targeted to late endosomes via the sorting signals in the cytoplasmic tail of the M6PR. However, phosphorylation of U21 is undetectable in U21-expressing cells, suggesting that U21 is not mannose 6-phosphorylated (18). Further, there exists a genetic disease in humans characterized by the absence of the enzyme necessary for mannose 6-phosphorylation of lysosomal hydrolases, called inclusion cell (I-cell) disease, which is predictably characterized by secretion of lysosomal hydrolases. We reasoned that if mannose 6-phosphorylated glycans on U21 act as the targeting signal for U21-mediated trafficking of class I molecules to lysosomes, then in U21-expressing I-cells, class I MHC molecules should be unaffected by the presence of U21 and remain on the cell surface. However, expression of U21 in I-cells resulted in diversion of class I MHC molecules to the lysosomal compartment (data not shown), demonstrating that mannose 6-phosphorylation is not important for U21-mediated lysosomal targeting. It is also possible that the M6PR itself is protein X, associating with U21 independent of the mannose 6-phosphorylation status of its glycans. To test this possibility, we knocked down the CI-M6PR and found that the ability of U21 to redirect class I molecules to lysosomes is unaffected (data not shown). It remains possible that the CD-M6PR is protein X. If so, however, we have thus far failed to retrieve a polypeptide of appropriate size in coimmunoprecipitation experiments.
We do not yet know whether the class I-U21 complex travels to the lysosomal compartment directly from the secretory pathway or via internalization from the plasma membrane. We have been unable to surface-biotinylate U21 or class I MHC molecules in cells expressing U21. Normally, class I MHC molecules are phosphorylated on their cytoplasmic tails upon arrival at or internalization from the plasma membrane (36). However, in cells expressing U21, we detect no phosphorylated class I MHC molecules (18). Thus, although we cannot rule out the possibility that the amount of time spent by class I molecules at the cell surface is too fleeting to measure, these results suggest that class I and U21 proceed to the lysosomal compartment from the secretory pathway.
If our model is correct, and U21 associates with class I molecules and a cellular protein X, we next hypothesized that we might be able to create a mutant U21 molecule that could retain its ability to bind to class I MHC molecules but impair its ability to bind to protein X. In this case, U21 and class I molecules should maintain their association, but class I molecules should not be redirected to the lysosomal compartment. We first considered the possibility that the N-linked glycans might be involved in recognition of U21 by protein X. We found that mutation of the single conserved N-linked glycan on HHV-6A U21—N164Q—resulted in exactly this phenotype. The N164Q molecules maintained their association with class I molecules, but class I molecules were largely present at the cell surface and not in the lysosomal compartment. At the outset of these studies, our goal was to utilize the mutant U21 molecules as a negative control in coimmunoprecipitation/tandem affinity purification experiments: could we coimmunoprecipitate a polypeptide (protein X) that associates with WT U21SBPHA and class I molecules, but not with the N164Q U21SBPHA and class I molecules, and could we then identify protein X using mass spectroscopy? We have performed these experiments, but we have not yet discovered the identity of protein X. It is possible that the interaction between U21 and protein X is brief: as depicted in Fig. 14a (green protein X), protein X may associate with U21 and class I molecules in the trans-Golgi and mediate only the short trip to the endosomal compartment before dissociating. Alternatively, the interaction between protein X and U21 may be difficult to preserve, even under gentle detergent solubilization conditions. Thus, although we have made inroads toward understanding the mechanism of U21-mediated rerouting of class I MHC molecules to the endolysosomal compartment, identification of protein X still awaits.
Acknowledgments
We thank Lisa Kimpler for critical reading of the manuscript and Drs. Domenico Tortorella, Hidde Ploegh, Peter Cresswell, and Tom August for antibodies and reagents.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01AI069099 (to A. W. H.).
- HHV
- human herpesvirus
- ER
- endoplasmic reticulum
- β2m
- β2-microglobulin
- PNGase F
- N-glycosidase F
- endoH
- endoglycosidase H
- M6PR
- mannose 6-phosphate receptor
- shβ2m
- small hairpin RNA directed against β2m
- MCMV
- murine cytomegalovirus.
REFERENCES
- 1.Dewhurst S., Skrincosky D., van Loon N. (1997) Expert Rev. Mol. Med. 1997, 1–17 [DOI] [PubMed] [Google Scholar]
- 2.Zerr D. M. (2006) Herpes 13, 20–24 [PubMed] [Google Scholar]
- 3.Ward K. N. (2005) Curr. Opin. Infect. Dis. 18, 247–252 [DOI] [PubMed] [Google Scholar]
- 4.Tortorella D., Gewurz B. E., Furman M. H., Schust D. J., Ploegh H. L. (2000) Annu Rev. Immunol. 18, 861–926 [DOI] [PubMed] [Google Scholar]
- 5.Ahn K., Meyer T. H., Uebel S., Sempé P., Djaballah H., Yang Y., Peterson P. A., Früh K., Tampé R. (1996) EMBO J. 15, 3247–3255 [PMC free article] [PubMed] [Google Scholar]
- 6.Boname J. M., de Lima B. D., Lehner P. J., Stevenson P. G. (2004) Immunity 20, 305–317 [DOI] [PubMed] [Google Scholar]
- 7.Hengel H., Koopmann J. O., Flohr T., Muranyi W., Goulmy E., Hämmerling G. J., Koszinowski U. H., Momburg F. (1997) Immunity 6, 623–632 [DOI] [PubMed] [Google Scholar]
- 8.Ahn K., Angulo A., Ghazal P., Peterson P. A., Yang Y., Früh K. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10990–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coscoy L., Ganem D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8051–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glosson N. L., Hudson A. W. (2007) Virology 365, 125–135 [DOI] [PubMed] [Google Scholar]
- 11.Hudson A. W., Howley P. M., Ploegh H. L. (2001) J. Virol. 75, 12347–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishido S., Wang C., Lee B. S., Cohen G. B., Jung J. U. (2000) J. Virol. 74, 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones T. R., Wiertz E. J., Sun L., Fish K. N., Nelson J. A., Ploegh H. L. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 11327–11333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reusch U., Muranyi W., Lucin P., Burgert H. G., Hengel H., Koszinowski U. H. (1999) EMBO J. 18, 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson P. G., Efstathiou S., Doherty P. C., Lehner P. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8455–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiertz E. J., Jones T. R., Sun L., Bogyo M., Geuze H. J., Ploegh H. L. (1996) Cell 84, 769–779 [DOI] [PubMed] [Google Scholar]
- 17.Ziegler H., Thale R., Lucin P., Muranyi W., Flohr T., Hengel H., Farrell H., Rawlinson W., Koszinowski U. H. (1997) Immunity 6, 57–66 [DOI] [PubMed] [Google Scholar]
- 18.Hudson A. W., Blom D., Howley P. M., Ploegh H. L. (2003) Traffic 4, 824–837 [DOI] [PubMed] [Google Scholar]
- 19.Mostoslavsky G., Fabian A. J., Rooney S., Alt F. W., Mulligan R. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16406–16411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Call M. E., Pyrdol J., Wiedmann M., Wucherpfennig K. W. (2002) Cell 111, 967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blom D., Hirsch C., Stern P., Tortorella D., Ploegh H. L. (2004) EMBO J. 23, 650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelham H. R. (1988) EMBO J. 7, 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson M. R., Nilsson T., Peterson P. A. (1993) J. Cell Biol. 121, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson M. R., Nilsson T., Peterson P. A. (1990) EMBO J. 9, 3153–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro S., Pelham H. R. (1987) Cell 48, 899–907 [DOI] [PubMed] [Google Scholar]
- 26.Nilsson T., Jackson M., Peterson P. A. (1989) Cell 58, 707–718 [DOI] [PubMed] [Google Scholar]
- 27.Little A. M., Nössner E., Parham P. (1995) J. Immunol. 154, 5205–5215 [PubMed] [Google Scholar]
- 28.von Heijne G. (1989) Nature 341, 456–458 [DOI] [PubMed] [Google Scholar]
- 29.Boname J. M., Thomas M., Stagg H. R., Xu P., Peng J., Lehner P. J. (2009) Traffic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartee E., Mansouri M., Hovey Nerenberg B. T., Gouveia K., Früh K. (2004) J. Virol. 78, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May N. A., Glosson N. L., Hudson A. W. (2010) J. Virol. 84, 3738–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chikh K., Vey S., Simonot C., Vanier M. T., Millat G. (2004) Mol. Genet. Metab. 83, 220–230 [DOI] [PubMed] [Google Scholar]
- 33.Ihrke G., Bruns J. R., Luzio J. P., Weisz O. A. (2001) EMBO J. 20, 6256–6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vagin O., Turdikulova S., Sachs G. (2004) J. Biol. Chem. 279, 39026–39034 [DOI] [PubMed] [Google Scholar]
- 35.Reusch U., Bernhard O., Koszinowski U., Schu P. (2002) Traffic 3, 752–761 [DOI] [PubMed] [Google Scholar]
- 36.Eichholtz T., Vossebeld P., van Overveld M., Ploegh H. L. (1992) J. Biol. Chem. 267, 22490–22495 [PubMed] [Google Scholar]