FIGURE 5.
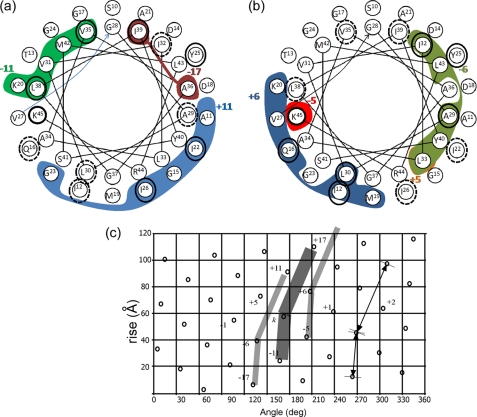
Correlation of the measured shift perturbations Δδ and a 3.5-Å intersubunit interface in the low temperature form of Pf1, model 4IFM. A helical representation of residues Ser-10 to Lys-45 is shown. The internal circle represents the C-terminal residues Gly-28–Lys-45. The external circle is for Ser-10–Val-27. The colored areas enclose amino acids for which at least one carbon or nitrogen is within 3.5 Å of a neighboring subunit in the model, and the identity of this subunit is indicated by a colored number. a, contacts with subunits +11 (blue), −11 (green), and −17 (red) are shown (for +17 and only contacts with Ile-3 exist). b, contacts with subunits +6 (blue), −6 (green), and the two residues of subunit ±5 (orange and red) are shown. Amino acids circled in black show Δδ ≥0.4 ppm in our data. The dashed circles in a correspond to shifts in van der Waals contact areas of the subunits emphasized in b and vice versa. See text for further details. c, a lattice diagram corresponding to the low temperature symmetry of the Pf1 filamentous bacteriophage. A rotation of 65.9° and rise of 3.05 Å were used to generate the dots. A representative subunit k is sketched as a broad bent line, and the neighboring subunits ±6 are indicated by narrower lines. Subunits in contact with subunit k are numbered according to a right-handed sense of the Pf1 capsid helix.