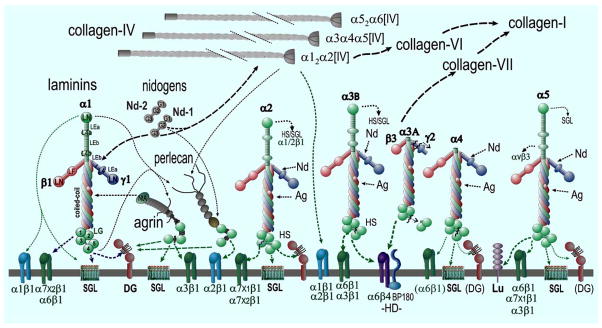
Fig. (1). Basement Membrane Components and Interactions.
Examples are shown for major laminins. Each possesses three subunits and possesses globular, rod-like and coiled-coil domains (these are indicated for laminin-111). Various proteolytic cleavages (jagged lines) generate additional diversity. Each type IV collagen is a heterotrimer with three different chain compositions (most common is α12α2[IV]). In addition are found two nidogens (Nd1, Nd2), and the heparan sulfate proteoglycans agrin (Ag) and perlecan (perl). Major binding and minor (heavy and thin dashed arrows) binding interactions are indicated among the structural components (black lines) and with cell surface components (green lines to integrins, α-dystroglycan (αDG), hemidesmosomal (HD) BP180, sulfated glycolipids (SGL) and the Lutheran (Lu) antigen. In squamous epithelia, laminin 3A32 is processed by several proteases. It binds to the α6β4 integrin and BP180 and binds to type VII collagen.