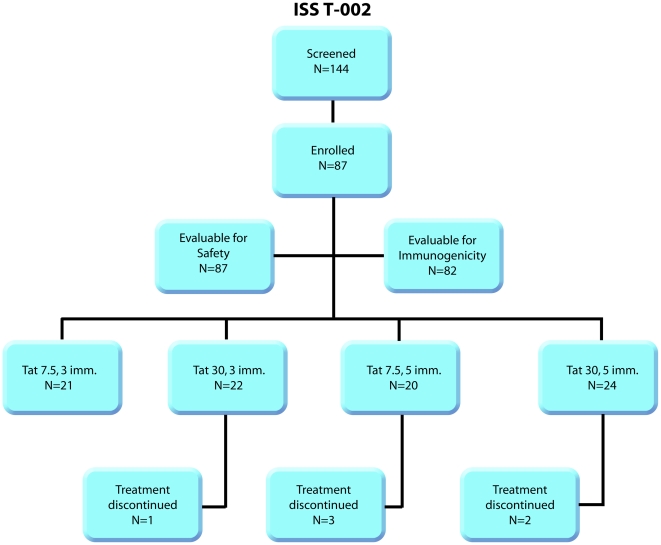
Figure 1. Flow diagram of the study participants.
One hundred and forty-four HAART-treated patients were screened for enrollment. Of them, 87 met the inclusion criteria and were randomized on schedule and dose of immunization. This represents all the study population prior to the protocol amendment. All recruited individuals were included in the safety analysis (n = 87). Six subjects discontinued the immunization schedule. Of them, 5 were evaluated only for safety, since received at least one immunization, and 1 was evaluated also for immunogenicity since received 3 immunizations out of 5 (as indicated in the Protocol S1). A total of 82 individuals completed the 20-weeks period of the study and 68 have completed the 48-weeks period after the first immunizations.