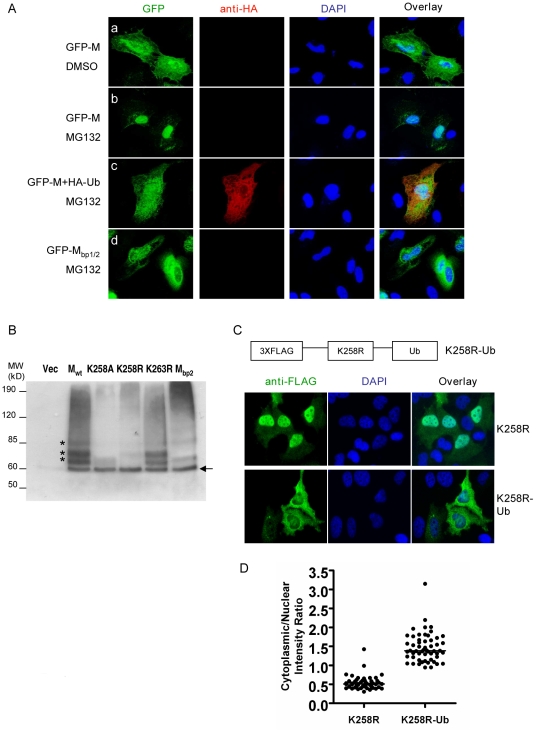
Figure 6. Ubiquitination regulates NiV-M nuclear export.
(A) Ubiquitin depletion by MG132 treatment inhibits M nuclear export. HeLa cells were transfected with GFP-M alone (panels a and b), GFP-M plus HA-Ub (panel c) or GFP-Mbp1/2 (panel d). 24 hpt, cells were treated with 50 µM MG132 or DMSO as indicated, fixed 6 hrs later with 2% paraformaldehyde, stained with DAPI as well as a mouse anti-HA antibody followed by Alexa594-conjugated goat-anti-mouse secondary antibody to identify cells expressing HA-Ub, and imaged on a fluorescent microscope. Representative images are shown. (B) Ubiquitination patterns of wild-type M and the indicated mutants. HEK293T cells were co-transfected with HA-Ub (in which all the lysines were mutated to arginines to specifically look at monoubiquitination) and the indicated 3XFLAG-tagged M mutants or empty vector as control. M was immunoprecipitated as described in Materials and Methods and the ubiquitinated species were detected by immunoblotting using an anti-HA antibody. The banding patterns of K258A, K258R and Mbp2 were different from Mwt, whereas K263R was similar to Mwt. (C) Mimicking monoubiquitination restores nuclear export to K258R. One copy of ubiquitin was fused in frame to the C-terminus of 3XFLAG-K258R, and HeLa cells expressing K258R or K258R-Ub were stained with an anti-FLAG antibody. Quantification of the cytoplasmic/nuclear fluorescence intensity ratio for each mutant is shown in (D). There is significant difference between the localization patterns of K258R and K258R-Ub (p<0.0001, unpaired t test).