Abstract
Cannabis use during adolescence is associated with an increased risk for schizophrenia and other disorders. The neuronal basis is unclear, but prefrontal cortical mechanisms have been implicated. Here, we investigated developmental changes in the endocannabinoid system by assessing expression and function of the CB1 cannabinoid receptor in prefrontal and other cortical areas in juvenile (postnatal day 25, P25), adolescent (P40) and adult (P70) rats. Overall, the expression of CB1 receptors in the cortex is highest in juveniles and drops thereafter towards adult levels. However, CB1 receptor expression follows distinct developmental trajectories in different cortical areas. The most pronounced and progressive decrease in CB1 expression was observed in medial prefrontal and other limbic/associative regions. In contrast, major changes in sensorimotor cortices occurred only after P40. We also assessed electrophysiological measures of CB1 receptor function and found that CB1-dependent inhibition of synaptic transmission in the prefrontal cortex follows the same developmental trajectory as observed for receptor expression. Together, these findings indicate that CB1 receptor-mediated signaling decreases during development, but is differentially regulated in limbic/associative vs. sensorimotor systems. Therefore, cannabis use during adolescence likely differentially affects limbic/associative and sensorimotor cortical circuits.
Keywords: adolescence, CB1 cannabinoid receptor, gene expression, prefrontal cortex
Introduction
Converging evidence indicates that adolescence is a vulnerable period for the onset of several neuropsychiatric disorders, including schizophrenia and drug addiction (Andersen, 2003; Chambers et al., 2003; Leslie et al., 2004; O'Brien and Anthony, 2005). For example, symptoms of schizophrenia often first occur during adolescence (Weinberger, 1987; Harrison and Weinberger, 2005; Do et al., 2009). However, little is known about the neurodevelopmental processes that underlie this vulnerability. Recent epidemiological findings implicate the endocannabinoid system by showing a strong association between cannabis use during adolescence and an increased risk for schizophrenia (Caspi et al., 2005; Henquet et al., 2005; Moore et al., 2007).
Endocannabinoids are retrograde neuromodulators that regulate the strength of excitatory and inhibitory synaptic transmission in neural circuits, primarily via activation of the CB1 cannabinoid receptor (Harkany et al., 2008). The CB1 receptor is also responsible for the effects of the psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (Devane et al., 1988). However, the neural circuits that mediate the different actions of cannabis are not fully understood (Realini et al., 2009). Long-term use of cannabis is associated with deficits in working memory and decision making (Solowij et al., 2002), which are dependent on prefrontal cortex functioning (Casey et al., 2000; Spear, 2000; Luna et al., 2004; Segalowitz and Davies, 2004; Bunge and Wright, 2007). The CB1 cannabinoid receptor is highly expressed in the prefrontal cortex (Bodor et al., 2005; Eggan and Lewis, 2007), and this cortical region undergoes widespread structural and molecular refinements during the adolescent transition period (Woo et al., 1997; Casey et al., 2000; Spear, 2000; Andersen, 2003; Gogtay et al., 2004; Pantelis et al., 2005). Thus, elucidating developmental changes in CB1 expression and function during the periadolescent maturation of prefrontal cortical circuits should further our understanding of the role of the endocannabinoid system in this period of vulnerability. In the present study, we used in situ hybridization histochemistry to assess developmental changes in CB1 expression in the prefrontal cortex and to compare them with those in other cortical areas. Age-dependent changes in CB1 receptor function in the prefrontal cortex were determined by electrophysiological and pharmacological approaches.
Methods and Materials
Subjects
Experiments were conducted in male Sprague–Dawley rats (Harlan, Madison, WI, USA), which were housed in groups of 2–4 per cage and maintained on a 12 h light/dark cycle with food and tap water available ad libitum. CB1 mRNA expression was studied at postnatal days 25 (P25), 40 (P40) and 70 (P70) (n=6 each). These animals were allowed to habituate for 3 days after arrival before they were killed for tissue processing. Electrophysiological measures of CB1 receptor function were assessed in two age groups: juvenile/preadolescent (P28–35) and adult (P60–75). All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Tissue preparation and in situ hybridization histochemistry
The rats were killed with CO2, and their brain was rapidly removed, frozen in isopentane cooled on dry ice and then stored at −30 °C until cryostat sectioning. Twelve μm thick coronal sections were thaw-mounted onto glass slides (Superfrost/Plus, Daigger, Wheeling, IL, USA) and dried on a slide warmer. During in situ hybridization histochemistry, the tissue of all animals was processed together. The sections were first fixed in 4% paraformaldehyde/0.9% saline for 10 min at room temperature, incubated in a fresh solution of 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% saline (pH 8.0) for 10 min, dehydrated, defatted for 2 × 5 min in chloroform, rehydrated, and air-dried. The slides were then stored at −30 °C until hybridization.
The oligonucleotide probe (48-mer; Invitrogen, Rockville, MD, USA) was labeled with [35S]-dATP as described earlier (Steiner and Kitai, 2000). The probe was complementary to bases 1051–1098 of the CB1 mRNA (GenBank accession number X55812). One hundred μl of hybridization buffer containing labeled probe (~3 × 106 cpm) was added to each slide. The sections were coverslipped and incubated at 37 °C overnight. After incubation, the slides were first rinsed in four washes of 1× saline citrate (150 mM sodium chloride, 15 mM sodium citrate), and then washed 3 times 20 min each in 2× saline citrate/50% formamide at 40 °C, followed by 2 washes of 30 min each in 1× saline citrate at room temperature. After a brief water rinse, the sections were air-dried and then apposed to X-ray film (BioMax MR-2, Kodak) for 3 days.
Analysis of autoradiograms
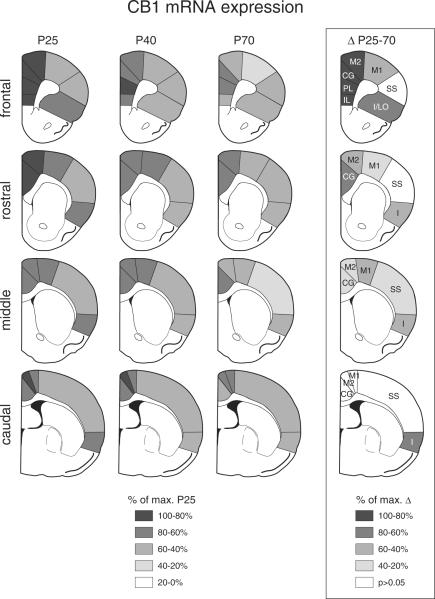
Gene expression was assessed in sections from 4 rostrocaudal levels (frontal, approximately at +2.7 mm relative to bregma; rostral, +1.6; middle, +0.4; caudal, −0.8; see Figure 2), in a total of 22 cortical regions (from medial to lateral) (Paxinos and Watson, 1998): cingulate, medial agranular (M2), motor (M1), somatosensory and insular cortex on frontal to caudal levels, and infralimbic, prelimbic and insular/lateral orbital cortex on the frontal level. Hybridization signals on film autoradiograms were measured by densitometry (NIH Image; Wayne Rasband, NIMH, Bethesda, MD, USA). The film images were captured using a light table (Northern Light, Imaging Research, St. Catharines, Ontario, Canada) and a Sony CCD camera (Imaging Research). The “mean density” value of a region of interest was measured by placing a template over the captured image. Mean densities were corrected for background by subtracting mean density values measured over white matter (corpus callosum). Values from corresponding regions in the two hemispheres were then averaged. Treatment effects were determined by one- or two-factor ANOVA, followed by Newman-Keuls post hoc tests to describe differences between individual groups (Statistica, StatSoft, Tulsa, OK, USA). For illustrations of topographies (maps), gene expression in a given region was expressed relative to the maximal value (% of max.) observed in the P25 group. The illustrations of film autoradiograms displayed in Figure 1 are computer-generated and contrast-enhanced images. Maximal hybridization signal is black.
Figure 2.
Regional distribution of CB1 receptor expression in the cortex at different postnatal ages. The maps illustrate the distribution of CB1 mRNA across different cortical regions on frontal, rostral, middle and caudal levels in juvenile (P25, n=6), adolescent (P40, n=6) and adult (P70, n=6) rats, as well as the differences (p<0.05) in CB1 expression between P25 and P70 animals (Δ P25–70). The values are expressed in percentage of the maximal value in the P25 group, or of the maximal difference observed, respectively, and are coded as indicated. IL, infralimbic; PL, prelimbic; CG, cingulate; M2, medial agranular; M1, motor; SS, somatosensory; I, insular.
Figure 1.
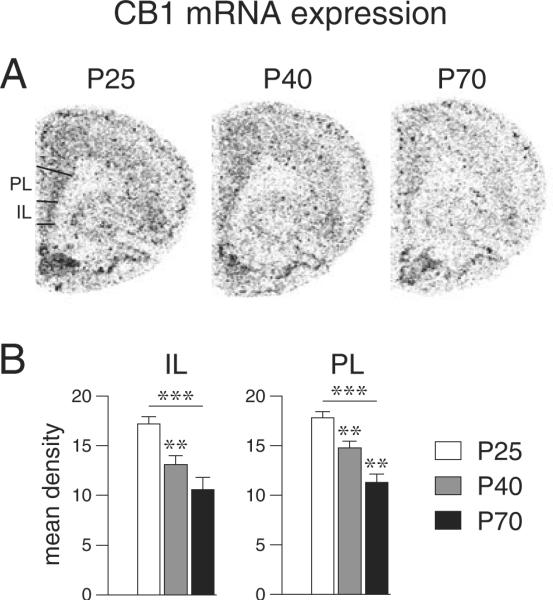
Developmental regulation of CB1 mRNA expression in the medial prefrontal cortex. (A) Illustrations of film autoradiograms depict expression of the CB1 receptor in the frontal cortex of juvenile (P25), adolescent (P40) and adult (P70) rats. (B) Mean density values (mean ± SEM) for CB1 receptor expression in the infralimbic (IL) and prelimbic (PL) regions are shown. CB1 mRNA levels were highest at P25. In both regions, levels were significantly reduced at P40 and fell further towards P70, when they reached ~60% of P25 levels. **P<0.01, ***P<0.001 vs. preceding age group or as indicated.
Electrophysiology
Slice preparation
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) before being decapitated (Tseng and O'Donnell, 2004). Brains were rapidly removed into ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): 125 NaCl, 25 NaHCO3, 2.5 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 3 MgCl2 and 0.05 picrotoxin (pH 7.40–7.43, 295–305 mOsm). Coronal slices (300 μm thick) containing infralimbic and prelimbic regions of the medial prefrontal cortex were obtained with a Vibratome (Pelco 102, Ted Pella, Redding, CA, USA) in ice-cold aCSF, and incubated in warm (35 °C) aCSF solution constantly oxygenated with 95% O2-5% CO2 for at least 60 min before recording. All chemicals and drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Whole-cell patch clamp recordings
All experiments were conducted at 33–35 °C. In the recording aCSF (delivered at 2 ml/min), CaCl2 was increased to 2 mM and MgCl2 was decreased to 1 mM. Patch electrodes (6–9 MΩ) were obtained from 1.5 mm borosilicate glass capillaries (World Precision Instruments, Sarasota, FL, USA) with a horizontal puller (P-97, Sutter Instruments, Novato, CA, USA) and filled with a solution containing 0.125% Neurobiotin and (in mM): 140 K-gluconate, 10 HEPES, 2 MgCl2, 3 Na2-ATP, 0.3 GTP (pH 7.3, 280–285 mOsm). Medial prefrontal cortex pyramidal neurons from layer V were identified under visual guidance using infrared (IR)-differential interference contrast video microscopy with a 40× water-immersion objective (Olympus BX51-WI, Olympus America, Center Valley, PA, USA). The image was detected with an IR-sensitive CCD camera and displayed on a monitor. Whole-cell patch-clamp recordings were performed with a computer-controlled amplifier (MultiClamp 700B, Molecular Devices, Sunnyvale, CA, USA), digitized (Digidata 1440, Molecular Devices, Sunnyvale, CA, USA) and acquired with Axoscope 10.1 (Molecular Devices, Sunnyvale, CA, USA) at a sampling rate of 10 KHz. The liquid junction potential was not corrected and electrode potentials were adjusted to zero before obtaining the whole cell configuration. The membrane potential was held at −70 mV in voltage-clamp mode throughout the experiment.
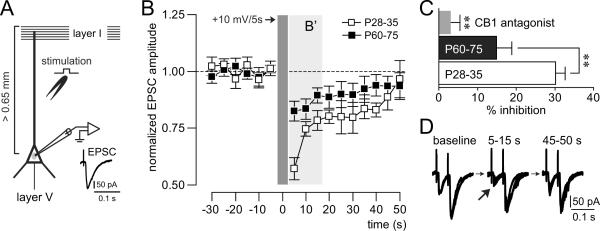
Electrically-evoked excitatory synaptic responses were elicited by local stimulation (0.05 to 0.15 mA square pulses of 0.3 ms duration delivered every 5 s) using a bipolar electrode made from a pair of twisted Teflon-coated nichrome wires (tips separated by approximately 150 μm) and placed around 300 μm lateral to the apical dendrite of the recorded neuron (Figure 5A). The intensity of stimulation was chosen from the minimum amount of current to elicit a synaptic response with <15% variability in amplitude during the first 10 min of recording. Only neurons that remained with such synaptic response reliability during the subsequent 15 min of baseline recording were included in the present study. If the current intensity required was >0.15 mA, the neuron was discarded. Input resistance and evoked synaptic responses were analyzed before and after drug application.
Figure 5.
(A) Recording arrangement designed to study depolarization-induced suppression of excitation (DSE) in deep-layer pyramidal neurons of the medial prefrontal cortex. DSE was assessed by measuring the effect of transient (5 s) postsynaptic depolarization (+10 mV) on electrically-evoked EPSCs elicited by local stimulation of excitatory inputs onto the apical dendrite. (B) Time course of evoked-EPSC amplitude recorded in pyramidal cells from juveniles (P28–35; n=7 cells/5 rats) and adults (P60–75; n=8 cells/6 rats) before, during and after DSE. The results show that the magnitude of DSE is more robust in the juvenile prefrontal cortex (B'). (C) Bar graph depicting the averaged amplitude inhibition in the first three measurements during DSE as shown in B' and the results of the CB1 receptor antagonist treatment. Bath application of the CB1 receptor antagonist AM-251 (4 μM) blocked the DSE in all cells tested (n=8 cells/8 rats). Group effect (P28–35 vs. P60–75, P28–35 vs. AM-251 and P60–75 vs. AM-251): **P<0.01. (D) Electrophysiological traces of evoked-EPSCs recorded before (baseline), during (5–15 s) and after (45–50 s) DSE. Note the reduction of the first EPSC amplitude (arrow) while the second EPSC remained unchanged.
Developmental regulation of CB1 receptor function was determined by measuring the magnitude of postsynaptic depolarization-induced suppression of excitation (DSE; Kreitzer and Regehr, 2002; Lovinger, 2008) in deep-layer pyramidal neurons of the medial prefrontal cortex. DSE was assessed by examining the effect of transient (5 s) postsynaptic depolarization (+10 mV) on electrically-evoked EPSCs elicited by local stimulation of excitatory inputs onto the apical dendrite.
Control and drug-containing aCSF were continuously oxygenated throughout the experiments. The CB1 antagonist AM-251 was purchased from Tocris Bioscience (Ellisville, MO, USA). All measures are expressed as mean ± SEM. Drug effects were compared using Student's t-test or repeated measures ANOVA, and the differences between experimental conditions were considered statistically significant when P<0.05.
Histology
All neurons included in the present study were labeled with Neurobiotin. After completion of the recording session, the slices were fixed with 10% formalin overnight at 4°C and stored in 0.1 M phosphate buffer (PB) until staining. After a series of rinses in 0.1 M PB, slices were incubated in 3% bovine serum albumin and 2% Triton-X 100 in PB for 1 hour followed by overnight in Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA, USA) at 4°C. Following another series of rinses, slices were reacted with 3,3' diaminobenzidine and urea-hydrogen peroxide (Sigma FAST DAB set). Slices were then rinsed, mounted on gelatin-coated slides, air-dried for 20 min, cleared in xylene, coverslipped in Permount and examined on a microscope.
Results
Age-dependent CB1 receptor expression in the medial prefrontal cortex
In the medial prefrontal cortex (frontal level), the expression of CB1 was assessed in the infralimbic and prelimbic regions (Figure 1). CB1 mRNA levels were highest at P25. In both regions, levels were significantly reduced at P40 and fell further towards P70, when they reached approximately 60% of P25 levels (Figure 1, Table 1).
Table 1.
Developmental regulation of CB1 mRNA expression in the cortex.
| P25 | P40 (% of P25) | P70 (% of P25) | ||
|---|---|---|---|---|
| FRONTAL | IL | 17.2 ± 0.7 | 13.1 ± 0.9 (76.2)** | 10.6 ± 1.2 (61.7)*** |
| PL | 17.8 ± 0.6 | 14.8 ± 0.6 (83.1)** | 11.3 ± 0.8 (63.8)***†† | |
| CG | 17.5 ± 0.8 | 13.6 ± 0.6 (77.6)** | 11.0 ± 0.6 (62.9)***† | |
| M2 | 14.8 ± 0.8 | 11.6 ± 0.7 (78.5)** | 9.3 ± 0.6 (62.7)***† | |
| M1 | 10.5 ± 0.6 | 9.3 ± 0.5 (88.9) | 6.9 ± 0.9 (65.9)**† | |
| SS | 9.8 ± 0.8 | 8.6 ± 0.4 (88.0) | 7.2 ± 1.1 (73.5) | |
| LO | 11.3 ± 0.5 | 9.5 ± 0.6 (83.8) | 7.3 ± 1.2 (64.7)** | |
| ROSTRAL | CG | 16.5 ± 0.6 | 13.8 ± 0.3 (83.3)** | 11.7 ± 0.5 (70.7)***†† |
| M2 | 14.4 ± 0.5 | 13.3 ± 0.8 (92.5) | 11.3 ± 0.5 (78.6)* | |
| M1 | 12.3 ± 0.7 | 11.3 ± 0.7 (91.9) | 9.8 ± 0.3 (79.7)* | |
| SS | 9.4 ± 0.7 | 9.3 ± 0.6 (99.1) | 7.4 ± 0.4 (78.8)† | |
| I | 11.2 ± 0.8 | 9.8 ± 0.6 (87.3) | 10.2 ± 0.5 (91.3)* | |
| MIDDLE | CG | 13.2 ± 0.6 | 11.9 ± 0.6 (90.5) | 10.7 ± 0.4 (81.3)* |
| M2 | 12.8 ± 0.7 | 13.3 ± 0.5 (103.9) | 10.4 ± 0.5 (81.6)*†† | |
| M1 | 11.7 ± 0.4 | 12.3 ± 0.4 (105.7) | 8.8 ± 0.5 (75.4)***††† | |
| SS | 8.7 ± 0.3 | 9.4 ± 0.5 (108.7) | 6.7 ± 0.4 (77.3)**††† | |
| I | 11.3 ± 0.5 | 10.3 ± 0.8 (91.9) | 8.5 ± 0.4 (75.6)* | |
| CAUDAL | CG | 12.2 ± 0.3 | 11.1 ± 0.5 (91.1) | 10.2 ± 1.1 (83.6) |
| M2 | 15.7 ± 1.0 | 14.8 ± 0.6 (94.1) | 13.1 ± 1.1 (83.5) | |
| M1 | 13.4 ± 0.9 | 13.2 ± 0.4 (98.1) | 12.0 ± 1.0 (89.4) | |
| SS | 9.3 ± 0.6 | 8.6 ± 0.5 (92.8) | 7.7 ± 0.5 (82.9) | |
| I | 11.7 ± 1.0 | 8.1 ± 0.9 (69.3)** | 7.7 ± 0.5 (65.7)** | |
Mean density values (mean ± SEM) measured in cortical areas on frontal, rostral, middle and caudal levels are shown for juvenile (P25), adolescent (P40) and adult (P70) rats. Expression levels relative to juveniles (% of P25) are given in parentheses. Abbreviations: IL, infralimbic; PL, prelimbic; CG, cingulate; M2, medial agranular; M1, motor; SS, somatosensory; I, insular.
P<0.05,
P<0.01,
P<0.001, vs. P25;
P<0.05,
P<0.01,
P<0.001, vs. P40.
CB1 receptor expression across the cortex: regional variations
CB1 expression in the medial prefrontal cortex was compared with the expression in other cortical regions on the frontal and more caudal levels. Overall, CB1 expression was highest at P25 in most of the 22 cortical areas investigated. Similar to the medial prefrontal cortex, there was a statistically significant decrease in CB1 expression from P25 to P70 in 16 of the 22 regions (Figure 2, Table 1). This decrease was most robust in the infralimbic, prelimbic, cingulate and medial agranular (M2) areas on the frontal level (Figure 2) and was generally weakest in motor and somatosensory cortical areas, especially on caudal levels (Figures 2 & 3).
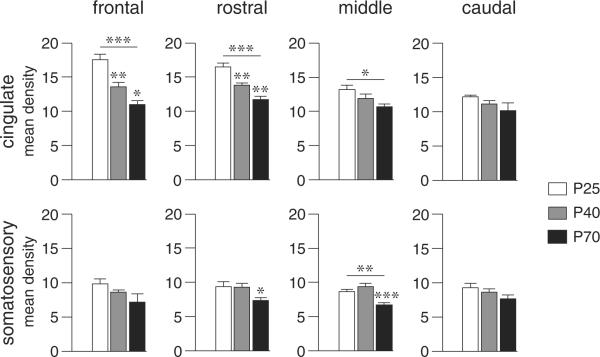
Figure 3.
CB1 receptor expression in the cingulate and somatosensory cortex at different postnatal ages. Mean density values (mean ± SEM) on the frontal, rostral, middle and caudal levels are given for juvenile (P25), adolescent (P40) and adult (P70) rats. *P<0.05, **P<0.01, ***P<0.001 vs. preceding age group or as indicated.
There was also a medial-lateral gradient in CB1 expression on all rostrocaudal levels, with highest expression medially in the cingulate and medial agranular cortex (M2) and lowest expression in the somatosensory cortex (Figures 2 & 3). This gradient was present at all ages, but was most distinctive at P25 (Figure 2). In some areas, there was also a rostrocaudal gradient, with higher expression rostrally. This effect was most prominent in the cingulate cortex at P25 (Figures 2 & 3)
Differential developmental trajectories of CB1 receptor expression in limbic/associative vs. sensorimotor areas
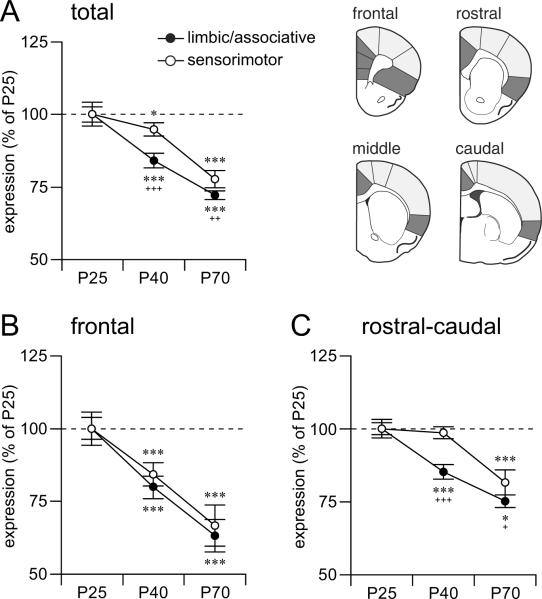
Comparison of the developmental trajectories in the different cortical areas revealed two distinct patterns. Similar to the medial prefrontal cortex (Figure 1), other areas of the limbic/associative cortex (cingulate, insular) on all 4 rostrocaudal levels displayed a progressive decrease in CB1 expression from P25 to P40 to P70 (Figures 2 & 3, Table 1). In contrast, CB1 expression in most sensorimotor areas (medial agranular -M2-, motor -M1-, somatosensory) remained unchanged between P25 and P40 (Figures 2 & 3), and showed a significant (but variable) decrease between P40 and P70. Pooling values from these functionally related areas (limbic/associative vs. sensorimotor) from all 4 rostrocaudal levels confirmed differential developmental trajectories (Figure 4). Thus, limbic/associative areas displayed significantly lower CB1 expression at each subsequent age, from P25 to P40 to P70, independent of the rostrocaudal level (Figure 4A). In contrast, sensorimotor areas showed a robust decrease only between P40 and P70 (Figure 4A). Consequently, at both P40 and P70, there was a significantly smaller reduction in expression in the sensorimotor areas compared with limbic/associative areas. This effect was seen with areas from all 4 rostrocaudal levels pooled (Figure 4A), but was minimal on the frontal level (Figure 4B). Thus, sensorimotor areas from the rostral-to-caudal levels mostly contributed to this differential trajectory (Figure 4C).
Figure 4.
Distinct developmental trajectories for CB1 receptor expression in limbic/associative vs. sensorimotor cortical regions. Expression level (in mean density, mean ± SEM; in % of P25) for CB1 mRNA in pooled limbic/associative (IL, PL, CG, I, I/LO; dark gray) and sensorimotor regions (M2, M1, SS; light gray) are given for (A) all 4 rostrocaudal levels pooled (total; main effect of age, F(2,15) = 20.5, P<0.001; main effect of region, F(1,15) = 26.6, P<0.001; age × region interaction, F(2,15) = 8.0, P<0.01), (B) the frontal level (age, F(2,15) = 12.3, P<0.001; region, F(1,15) = 3.9, P>0.05; age × region, F(2,15) = 1.0, P>0.05), and (C) pooled rostral-to-caudal levels (age, F(2,15) = 15.8, P<0.001; region, F(1,15) = 16.4, P<0.01; age × region, F(2,15) = 5.0, P<0.05). On the rostral-caudal levels, the limbic/associative regions displayed significantly reduced CB1 expression at both P40 and P70, whereas in the sensorimotor regions, a significant decrease was only observed at P70 (C). On the frontal level, both limbic/associative and sensorimotor regions exhibited similar trajectories (B). Age effects (P40 vs. P25 and P70 vs. P40): *P<0.05, **P<0.01, ***P<0.001. Region effects: +P<0.05, ++P<0.01, +++P<0.001.
CB1 receptor-mediated inhibition of excitatory synaptic transmission in the medial prefrontal cortex is age-dependent
To determine whether the developmental downregulation of cortical CB1 receptor expression (Figure 4) is associated with similar changes in CB1 receptor function, we conducted whole-cell patch clamp recordings and measured postsynaptic depolarization-induced suppression of excitation (DSE; +10 mV/5 s) in deep-layer pyramidal neurons of the medial prefrontal cortex (Figure 5A). DSE is a well-established electrophysiological response used to determine the endocannabinoid (CB1) regulation of synaptic activity (Kreitzer and Regehr, 2002; Lovinger, 2008). We found that depolarization-induced suppression of electrically-evoked excitatory postsynaptic currents (EPSCs) is markedly reduced in pyramidal neurons from adult (P60–75) prefrontal cortex as compared to that in juvenile/preadolescent (P28–35) rats (Figure 5B–C). As observed in other cortical regions (Fortin and Levine, 2007), DSE in the prefrontal cortex was transient and reversible (Figure 5B), and was associated with a facilitation of the paired-pulse ratio (PPR: EPSC2 / EPSC1) from 1.81 ± 0.07 to 2.67 ± 0.27 (p<0.01, paired t-Test) (Figure 5D). PPR is a sensitive measure for changes in neurotransmitter release, and a presynaptic mechanism underlying synaptic inhibition is often reflected in increased PPR (Thomson, 2000). To confirm that the synaptic inhibition observed in the prefrontal cortex is mediated by activation of CB1 receptors, we assessed the effect of the CB1 receptor antagonist AM-251 on DSE. Bath application of AM-251 (4 μM, n=8) significantly inhibited DSE (Figure 5C). Collectively, these electrophysiological and pharmacological data demonstrate that this CB1 receptor-dependent inhibition of synaptic activity is robust in juveniles and becomes attenuated in adults, indicating that CB1 receptor function in the prefrontal cortex is also developmentally regulated.
Discussion
In the present study, we assessed the expression of the CB1 receptor as a molecular marker for developmental changes in the endocannabinoid system in cortical circuits. Changes in CB1 receptor function were determined by means of electrophysiological measures combined with pharmacological manipulations. Overall, cortical CB1 expression and function were highest in juveniles and dropped thereafter towards adult levels. The most pronounced and progressive decrease in CB1 expression was observed in limbic/associative regions. In contrast, major changes in sensorimotor cortices occurred only after the adolescent transition period. Together, these results demonstrate a differential trajectory for CB1 receptor expression in limbic/associative vs. sensorimotor systems across postnatal development.
In the mammalian central nervous system, CB1 receptors are highly expressed in the forebrain (Herkenham et al., 1991; Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993) and are predominantly located presynaptically for both excitatory and inhibitory synapses (Harkany et al., 2008). The same is true in cortical circuits (Bodor et al., 2005; Fortin and Levine, 2007), particularly in layers II–III and V–VI where both pyramidal and non-pyramidal neurons express CB1 receptor mRNA (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993). A similar laminar distribution was found at all ages in the present study (not shown). Also consistent with previous studies (Herkenham et al., 1991; Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993), we observed a rostral-to-caudal gradient, with the highest CB1 expression in the prefrontal cortex. This gradient is also present at all ages, but is most distinctive at P25. Moreover, our present study revealed that there is also a medial-lateral gradient in CB1 expression on all rostrocaudal levels, with highest expression medially. Thus, CB1 receptors are most highly expressed in limbic/associative areas such as the prefrontal cortex and the cingulate cortex.
Our study demonstrates an age-dependent downregulation of cortical CB1 expression, an effect that is most distinctive in the prefrontal cortex. A similar downregulation of CB1 receptor density was observed in the prefrontal cortex during the periadolescent transition period (Ellgren et al., 2008), indicating that changes in cortical CB1 mRNA and protein levels follow the same developmental pattern. Our electrophysiological data expand upon these findings, by showing that CB1-dependent inhibition of synaptic activity in the prefrontal cortex follows the same developmental trajectory as CB1 expression, which is highest in juveniles and lowest in adults. Future studies will have to determine whether such a developmental regulation of CB1 receptor function is cell-type and/or synapse specific.
Despite the wealth of data stressing that endocannabinoids are critical for the normal formation of cortical networks during early/perinatal periods of development (Berghuis et al., 2007; Harkany et al., 2007; Harkany et al., 2008; Mulder et al., 2008), the role of endocannabinoid signaling at later postnatal stages of cortical maturation is currently unknown. It has been proposed that excessive stimulation of CB1 receptors during sensitive periods of postnatal development (e.g., adolescence) can produce enduring dysfunction in limbic/associative cortical circuits and increase the liability for schizophrenia (D'Souza et al., 2004; Caspi et al., 2005; D'Souza et al., 2005; Henquet et al., 2005; Moore et al., 2007; Realini et al., 2009). However, the impact of cannabis use during adolescence is likely not restricted to the limbic system. In addition to working memory and attention deficits, the effects of cannabis include alterations in sensory perception, impaired sensorimotor gating and hallucinations, all of which are indicators of dysfunctional information processing in sensorimotor circuits (D'Souza et al., 2004; D'Souza et al., 2005; Moore et al., 2007; Realini et al., 2009). Our finding of a lesser decrease of CB1 receptors in sensorimotor compared to limbic/associative areas during adolescence suggests that cannabis abuse during this transition period may have a relatively more robust impact on sensorimotor functions. Future studies are needed to determine the consequences of this differential regulation of CB1 receptor function during the periadolescent maturation in limbic/associative vs. sensorimotor regions.
Acknowledgements
This work was supported by Rosalind Franklin University of Medicine and Science (KYT) and National Institutes of Health Grants DA011261 (HS) and MH086507 (KYT).
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–330. doi: 10.1016/s0959-4388(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabas K, Ballester Rosado CJ, Nguyen L, Monory K, Marsicano G, Di Marzo V, Hurd YL, Guillemot F, Mackie K, Lutz B, Guzman M, Lu HC, Galve-Roperh I, Harkany T. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steiner H, Kitai ST. Regulation of rat cortex function by D1 dopamine receptors in the striatum. J Neurosci. 2000;20:5449–5460. doi: 10.1523/JNEUROSCI.20-14-05449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]