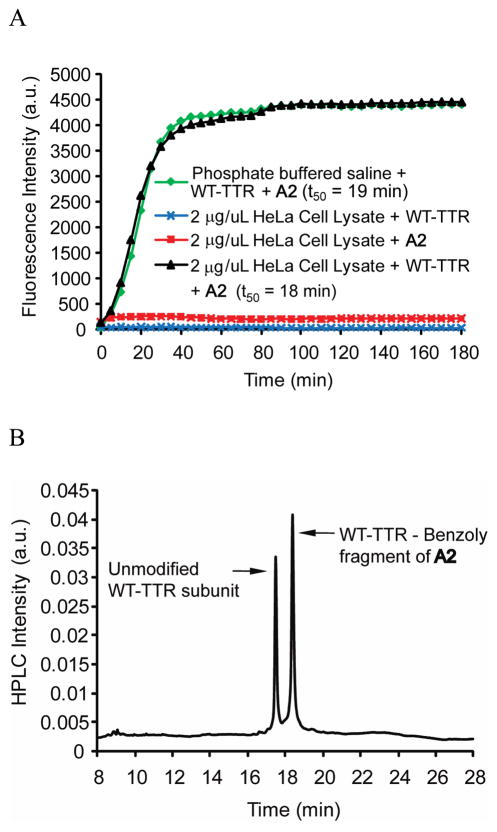
Figure 4.
Kinetics and chemoselectivity of the A2 conjugation reaction with WT-TTR in HeLa cell lysate vs. buffer. (A) Time-dependence of fluorescent conjugate formation, created by reacting A2 and WT-TTR in phosphate buffered saline (pH 7; green trace). Time-dependent fluorescence changes or lack thereof occurring when HeLa cell lysate (lacking TTR) is incubated with A2 alone (red trace) or when HeLa cell lysate is incubated alone with added TTR (without A2, blue trace) or when HeLa cell lysate with added WT-TTR is treated with A2 at 37 °C (black trace). (B) RP-HPLC analysis of TTR immunoisolated from HeLa cell lysate utilizing an anti-TTR antibody conjugated to resin. The nearly equal peak areas resulting from the unmodified subunits and the conjugated subunits (the molar absorptivity changes associated with benzoylation were accounted for) demonstrate high binding selectivity and a highly chemoselective amide bond forming reaction with TTR (49 % out of a maximum of 50 % of the subunits were modified).