Abstract
DNA molecules containing the 5' end of a functional major histocompatibility class II E alpha gene were injected into mouse eggs bearing E alpha genes with 630-base-pair (bp) deletions encompassing the promoter and first exon. The deletion was corrected by homologous recombination in 1 of about 500 transgenic mice that incorporated the injected DNA. The corrected E alpha gene was transmitted to progeny, which were bred to homozygosity. Southern blot analysis, polymerase chain reaction amplification of the DNA spanning the deletion, and sequence analysis revealed that the corrected allele resembles the wild-type E alpha gene. At sites of single-base-pair polymorphisms, there was apparently random conversion to either the donor or recipient sequence. In addition, many point mutations were introduced. mRNAs were produced from the corrected allele in a tissue-specific manner, but their sizes were different from the wild-type allele, and they did not produce detectable E alpha protein. This experiment demonstrates the feasibility of targeting foreign DNA to a gene that is completely inactive in fertilized mouse eggs.
Full text
PDF


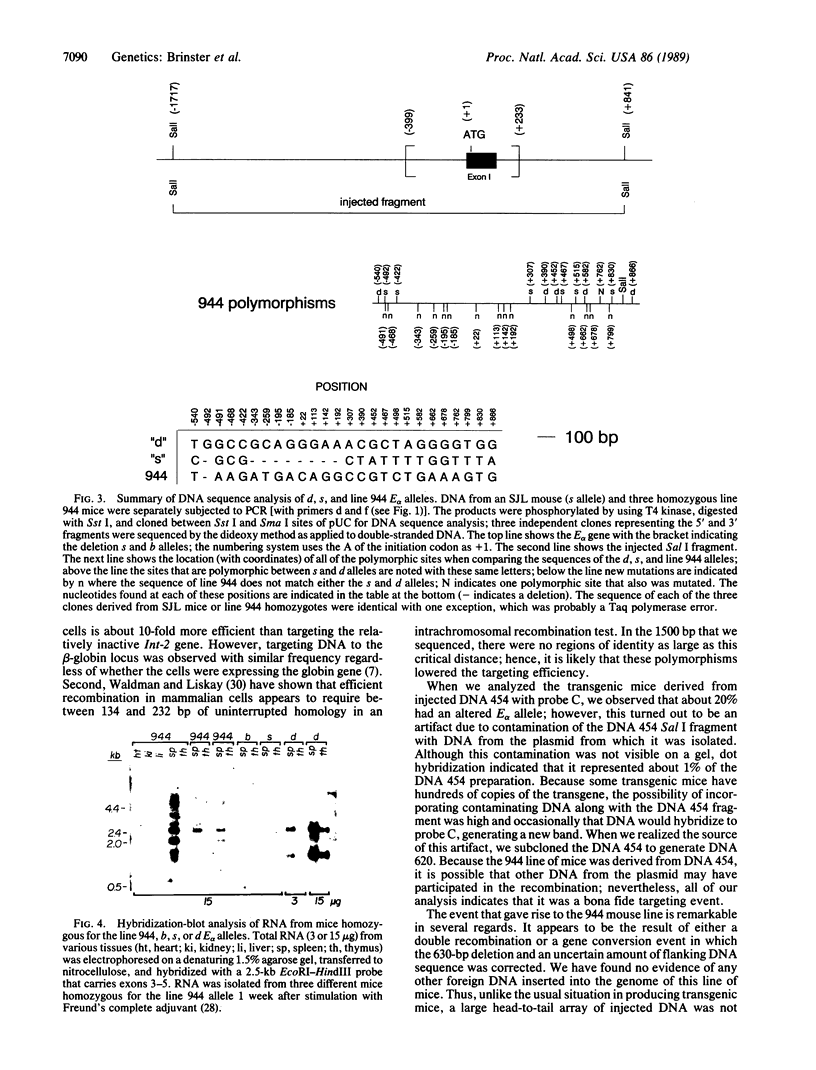

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Cowing C., Palmiter R. D., Brinster R. L., Flavell R. A. Selective expression of class II E alpha d gene in transgenic mice. J Immunol. 1989 Mar 15;142(6):2081–2088. [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Dembic Z., Ayane M., Klein J., Steinmetz M., Benoist C. O., Mathis D. J. Inbred and wild mice carry identical deletions in their E alpha MHC genes. EMBO J. 1985 Jan;4(1):127–131. doi: 10.1002/j.1460-2075.1985.tb02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Maeda N., Smithies O. Targeted mutation of the Hprt gene in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8583–8587. doi: 10.1073/pnas.85.22.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Durand B., Marfing C., Le Meur M., Benoist C., Mathis D. Conserved major histocompatibility complex class II boxes--X and Y--are transcriptional control elements and specifically bind nuclear proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6249–6253. doi: 10.1073/pnas.84.17.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A., Fehling H. J., Koch W., Le Meur M., Gerlinger P., Benoist C., Mathis D. B-cell control region at the 5' end of a major histocompatibility complex class II gene: sequences and factors. Mol Cell Biol. 1988 Oct;8(10):3975–3987. doi: 10.1128/mcb.8.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K., Thomas K., Capecchi M. R. Analysis of homologous recombination in cultured mammalian cells. Cold Spring Harb Symp Quant Biol. 1984;49:123–138. doi: 10.1101/sqb.1984.049.01.016. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Martin G. R. Cut, paste, and save: new approaches to altering specific genes in mice. Cell. 1989 Jan 27;56(2):145–147. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Schenning L., Hammerling U., Widmark E., Heldin E., Lind P., Servenius B., Lund T., Flavell R., Lee J. S. The complete nucleotide sequence of the I-E alpha d immune response gene. Nucleic Acids Res. 1983 Aug 11;11(15):5055–5071. doi: 10.1093/nar/11.15.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Berg P. Homologous integration in mammalian cells without target gene selection. Genes Dev. 1988 Nov;2(11):1353–1363. doi: 10.1101/gad.2.11.1353. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Skarnes W. C., Rossant J. Production of a mutation in mouse En-2 gene by homologous recombination in embryonic stem cells. Nature. 1989 Mar 9;338(6211):153–156. doi: 10.1038/338153a0. [DOI] [PubMed] [Google Scholar]
- Le Meur M., Gerlinger P., Benoist C., Mathis D. Correcting an immune-response deficiency by creating E alpha gene transgenic mice. Nature. 1985 Jul 4;316(6023):38–42. doi: 10.1038/316038a0. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Pinkert C. A., Widera G., Cowing C., Heber-Katz E., Palmiter R. D., Flavell R. A., Brinster R. L. Tissue-specific, inducible and functional expression of the E alpha d MHC class II gene in transgenic mice. EMBO J. 1985 Sep;4(9):2225–2230. doi: 10.1002/j.1460-2075.1985.tb03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Smithies O., Koralewski M. A., Song K. Y., Kucherlapati R. S. Homologous recombination with DNA introduced into mammalian cells. Cold Spring Harb Symp Quant Biol. 1984;49:161–170. doi: 10.1101/sqb.1984.049.01.019. [DOI] [PubMed] [Google Scholar]
- Subramani S., Berg P. Homologous and nonhomologous recombination in monkey cells. Mol Cell Biol. 1983 Jun;3(6):1040–1052. doi: 10.1128/mcb.3.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature. 1986 Nov 6;324(6092):34–38. doi: 10.1038/324034a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Thompson S., Clarke A. R., Pow A. M., Hooper M. L., Melton D. W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989 Jan 27;56(2):313–321. doi: 10.1016/0092-8674(89)90905-7. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol Cell Biol. 1988 Dec;8(12):5350–5357. doi: 10.1128/mcb.8.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera G., Burkly L. C., Pinkert C. A., Böttger E. C., Cowing C., Palmiter R. D., Brinster R. L., Flavell R. A. Transgenic mice selectively lacking MHC class II (I-E) antigen expression on B cells: an in vivo approach to investigate Ia gene function. Cell. 1987 Oct 23;51(2):175–187. doi: 10.1016/0092-8674(87)90145-0. [DOI] [PubMed] [Google Scholar]
- Wilkie T. M., Palmiter R. D. Analysis of the integrant in MyK-103 transgenic mice in which males fail to transmit the integrant. Mol Cell Biol. 1987 May;7(5):1646–1655. doi: 10.1128/mcb.7.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura K., Kikutani H., Folsom V., Clayton L. K., Kimoto M., Akira S., Kashiwamura S., Tonegawa S., Kishimoto T. Functional expression of a microinjected Ed alpha gene in C57BL/6 transgenic mice. Nature. 1985 Jul 4;316(6023):67–69. doi: 10.1038/316067a0. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]