Abstract
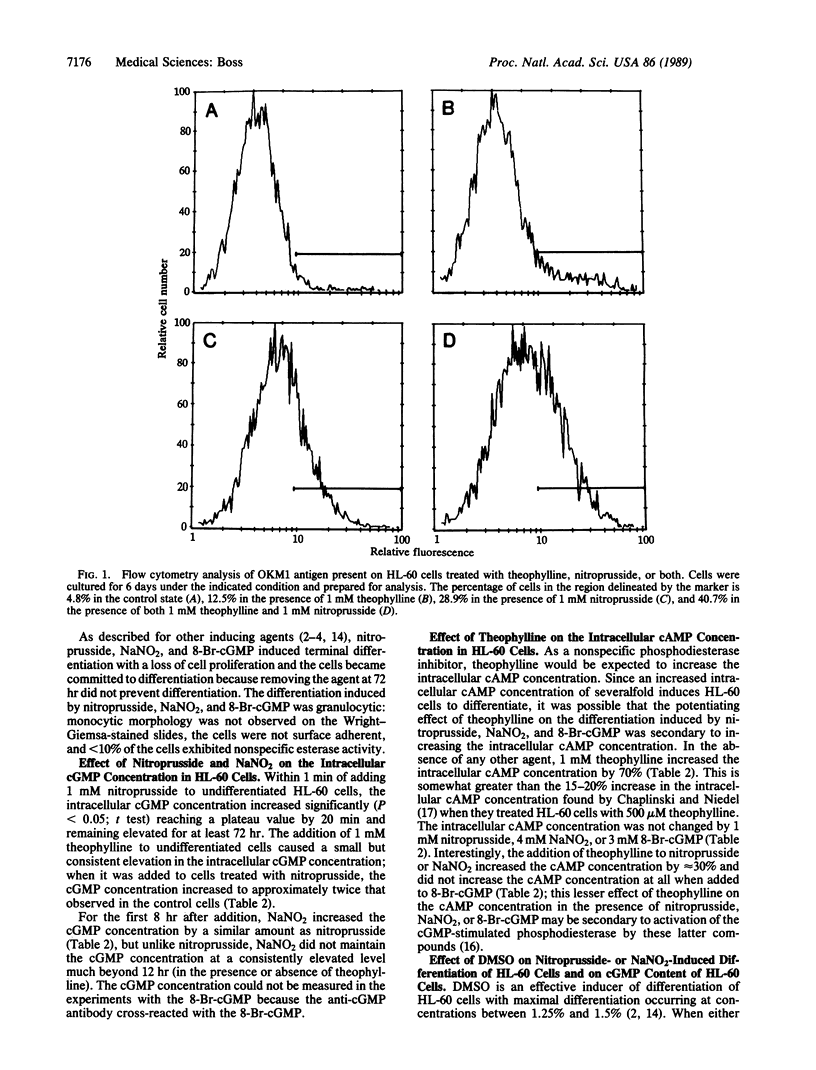
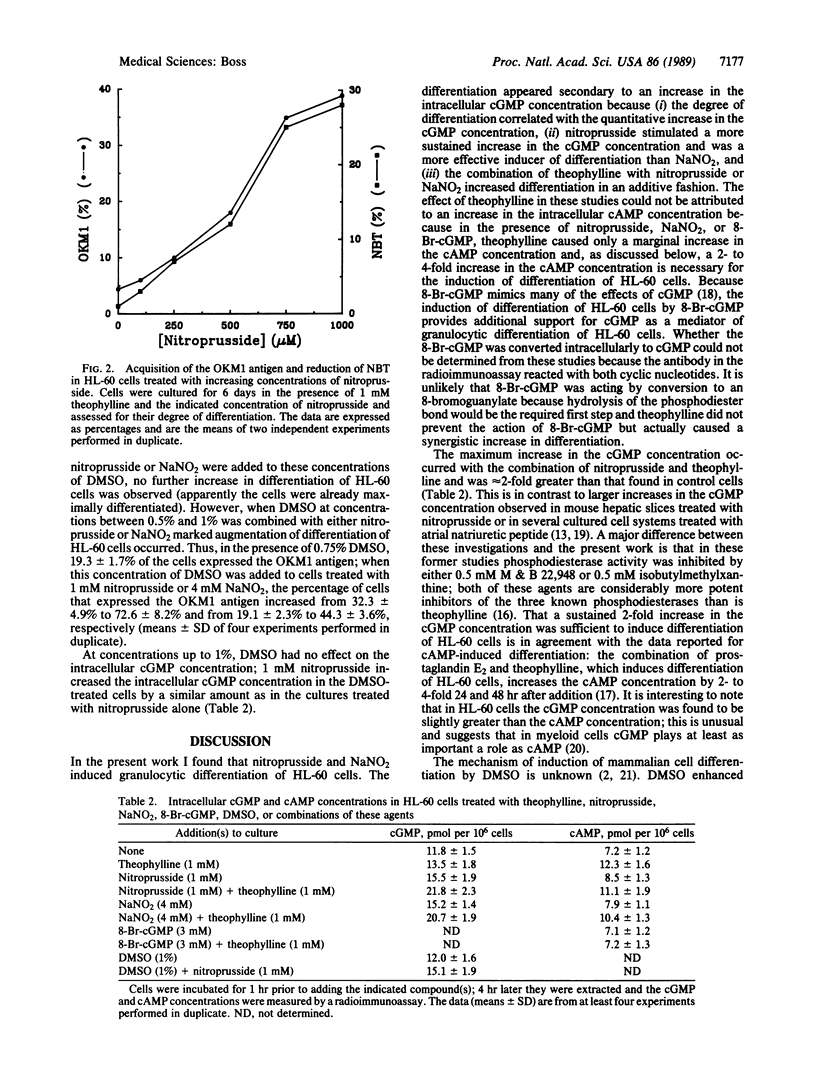
cGMP is a second messenger that mediates numerous metabolic events; in the present work a role in myeloid cell differentiation was demonstrated. Nitroprusside and NaNO2, which activate cytosolic guanylate cyclase and increase the intracellular cGMP concentration, induced granulocytic differentiation of the human promyelocytic cell line HL-60; differentiation was measured by acquisition of the OKM1 antigen, morphological changes, and nitroblue tetrazolium reduction. When theophylline, a phosphodiesterase inhibitor, which by itself induced modest differentiation, was added to nitroprusside or NaNO2, differentiation increased in an additive fashion. The degree of differentiation correlated with the increase in the intracellular cGMP concentration. 8-Bromoguanosine 3',5'-cyclic monophosphate, a membrane-permeable cGMP analogue, also induced differentiation of HL-60 cells but was much more effective in the presence of theophylline, with the two agents interacting synergistically. The effect of theophylline in these studies could not be attributed to increasing the intracellular cAMP concentration. Dimethyl sulfoxide, and established inducer of differentiation of HL-60 cells, markedly enhanced the differentiation induced by nitroprusside and NaNO2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplinski T. J., Niedel J. E. Cyclic AMP levels and cellular kinetics during maturation of human promyelocytic leukemia cells. J Leukoc Biol. 1986 Mar;39(3):323–331. doi: 10.1002/jlb.39.3.323. [DOI] [PubMed] [Google Scholar]
- Chaplinski T. J., Niedel J. E. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982 Nov;70(5):953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplinski T. J., Sloan G. J., Niedel J. E. Granulocyte functions during maturation of human promyelocytic leukemia cells. Leuk Res. 1985;9(7):897–903. doi: 10.1016/0145-2126(85)90311-x. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElDeib M. M., Parker C. D., Veum T. L., Zinn G. M., White A. A. Characterization of intestinal brush border guanylate cyclase activation by Escherichia coli heat-stable enterotoxin. Arch Biochem Biophys. 1986 Feb 15;245(1):51–65. doi: 10.1016/0003-9861(86)90189-x. [DOI] [PubMed] [Google Scholar]
- Ferrero D., Pessano S., Pagliardi G. L., Rovera G. Induction of differentiation of human myeloid leukemias: surface changes probed with monoclonal antibodies. Blood. 1983 Jan;61(1):171–179. [PubMed] [Google Scholar]
- Fibach E., Peled T., Rachmilewitz E. A. Self-renewal and commitment to differentiation of human leukemic promyelocytic cells (HL-60). J Cell Physiol. 1982 Oct;113(1):152–158. doi: 10.1002/jcp.1041130124. [DOI] [PubMed] [Google Scholar]
- Fontana J., Munoz M., Durham J. Potentiation between intracellular cyclic-AMP-elevating agents and inducers of leukemic cell differentiation. Leuk Res. 1985;9(9):1127–1132. doi: 10.1016/0145-2126(85)90102-x. [DOI] [PubMed] [Google Scholar]
- Friend C., Freedman H. A. Effects and possible mechanism of action of dimethylsulfoxide on Friend cell differentiation. Biochem Pharmacol. 1978 May 1;27(9):1309–1313. doi: 10.1016/0006-2952(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Koeffler H. P. Human acute myeloid leukemia lines: models of leukemogenesis. Semin Hematol. 1986 Jul;23(3):223–236. [PubMed] [Google Scholar]
- Kuno T., Andresen J. W., Kamisaki Y., Waldman S. A., Chang L. Y., Saheki S., Leitman D. C., Nakane M., Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986 May 5;261(13):5817–5823. [PubMed] [Google Scholar]
- Leitman D. C., Andresen J. W., Catalano R. M., Waldman S. A., Tuan J. J., Murad F. Atrial natriuretic peptide binding, cross-linking, and stimulation of cyclic GMP accumulation and particulate guanylate cyclase activity in cultured cells. J Biol Chem. 1988 Mar 15;263(8):3720–3728. [PubMed] [Google Scholar]
- Mato J. M., Krens F. A., van Haastert P. J., Konijn T. M. 3':5'-cyclic AMP-dependent 3':5'-cyclic GMP accumulation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2348–2351. doi: 10.1073/pnas.74.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Boswell K. H., Muneyama K., Simon L. N., Robins R. K., Shuman D. A. Synthesis and biochemical studies of various 8-substituted derivatives of guanosine 3',5'-cyclic phosphate, inosine 3',5'-cyclic phosphate, and xanthosine 3',5'-cyclic phosphate. Biochemistry. 1973 Dec 18;12(26):5310–5319. doi: 10.1021/bi00750a014. [DOI] [PubMed] [Google Scholar]
- Oshita A. K., Rothstein G., Lonngi G. cGMP stimulation of stem cell proliferation. Blood. 1977 Apr;49(4):585–591. [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pilz R. B., Van den Berghe G., Boss G. R. Induction of HL-60 differentiation by starvation for a single essential amino acid but not by protein synthesis inhibitors. J Clin Invest. 1987 Mar;79(3):1006–1009. doi: 10.1172/JCI112867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Taetle R., Koessler A. Effects of cyclic nucleotides and prostaglandins on normal and abnormal human myeloid progenitor proliferation. Cancer Res. 1980 Apr;40(4):1223–1229. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Wood K. S., Ignarro L. J. Hepatic cyclic GMP formation is regulated by similar factors that modulate activation of purified hepatic soluble guanylate cyclase. J Biol Chem. 1987 Apr 15;262(11):5020–5027. [PubMed] [Google Scholar]


