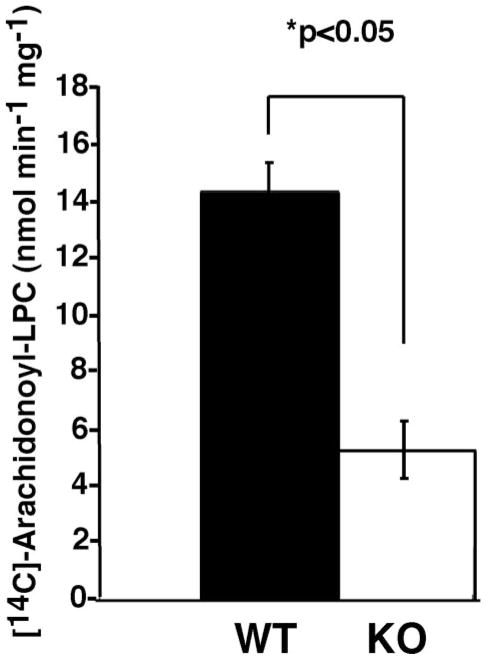
FIGURE 2. iPLA2γ activity in wild type and iPLA2γ knock-out mouse myocardial mitochondria.
Calcium-independent phospholipase A2γ has been previously demonstrated to selectively hydrolyze 1-palmitoyl-2-[1-14C]arachidonoyl phosphatidylcholine to generate 2-[1-14C]arachidonoyl LPC (12). To selectively measure iPLA2γ activity among other cellular mitochondrial phospholipases, mitochondria were isolated from WT and KO hearts, sonicated, and incubated in the presence of 1-palmitoyl-2-[1-14C]arachidonoyl phosphatidylcholine as described under “Experimental Procedures.” Remaining radiolabeled substrate and reaction products were extracted into butanol and resolved by TLC, and the amount of 2-[14C]arachidonoyl LPC was determined by scintillation spectrometry counting and expressed as nmol of 2-[14C]arachidonoyl LPC min−1 mg of mitochondrial protein−1. A dramatic 60% reduction in the production of 2-arachidonoyl LPC was observed in KO mitochondria in comparison with their WT counterparts (*, p < 0.05). All data were averaged from analyses of duplicate determinations from three mice and are shown as mean ± S.D.