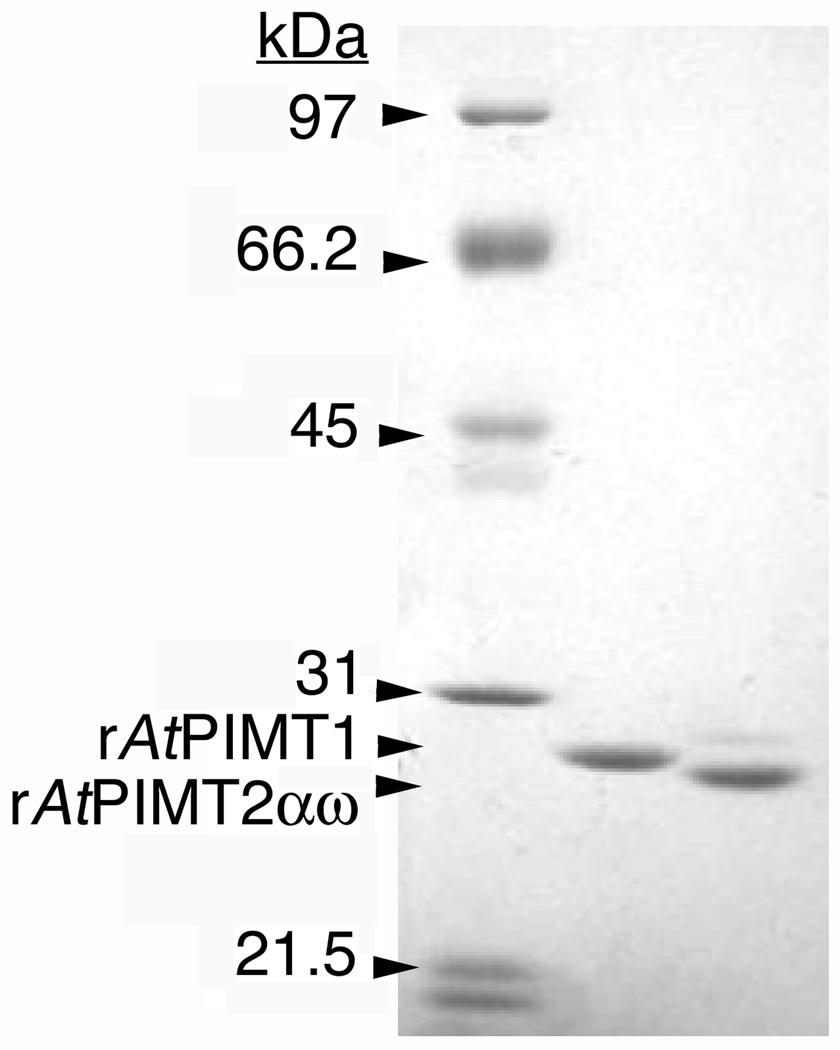
Figure 2.
Expression of recombinant protein repair methyltransfereases from Arabidopsis thaliana in E. coli. SDS-PAGE analysis of 2 µg of purified rAtPIMT1 protein (center lane) and 2 µg of purified rAtPIMT2αω protein (right lane) is shown. Gels contained 9.67 % acrylamide and 0.33 % N,N-methylene-bisacrylamide. Low range molecular mass standards (left lane; Bio-Rad, Hercules, CA) included phosphorylase a (97.4 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45 kDa), and carbonic anhydrase (31 kDa). The rAtPIMT1 and rAtPIMT2αω polypeptide bands migrated to positions corresponding to molecular masses of approximately 28.8 kDa and 28.1 kDa, respectively.