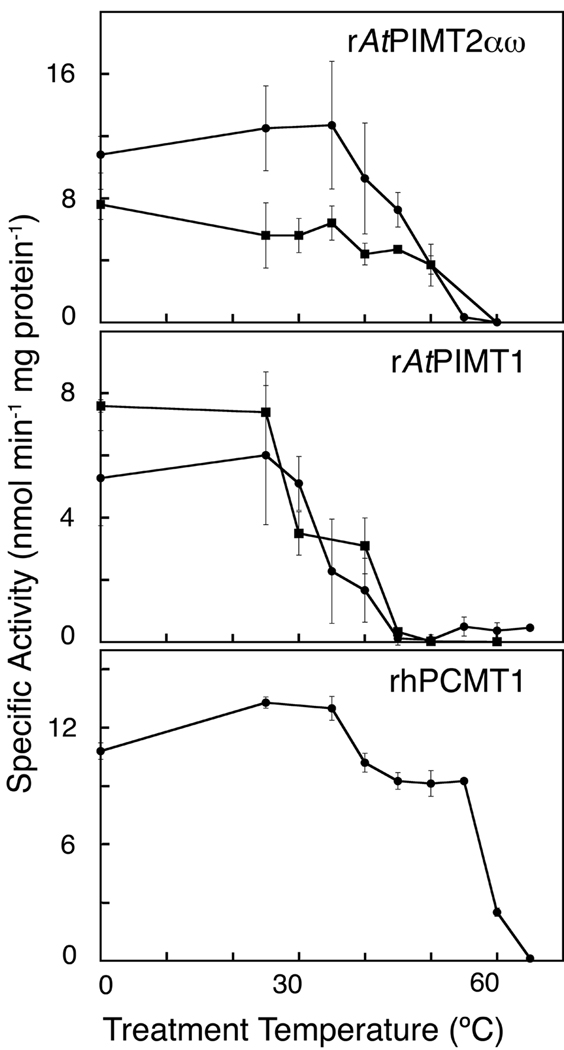
Figure 5.
Thermostability of recombinant Arabidopsis L-isoAsp methyltransferase. Purified recombinant enzyme was pretreated in elution buffer (λ; final pH of 8.0), or sodium phosphate buffer (ν; final pH of 6.9) over the temperature range of 0 °C to 65 °C (see Materials and Methods) for 10 min prior to its addition to the methyltransferase assay reaction mixture. Methyltransferase activity assays were performed for 10 min at 40 °C with rAtPIMT2αω (60 or 120 ng protein), rAtPIMT1 (70 ng protein), and rhPCMT (130 ng protein). The rAtPIMT2αω and rAtPIMT1 isozymes were purified in parallel and assayed in at least duplicate. rhPCMT was purified and studied separately. Error bars represent the standard deviation from the mean.