Abstract
Adiponectin promotes cardioprotection by various mechanisms, and this study used primary cardiomyocytes and the isolated working perfused heart to investigate cardiometabolic effects. We show in adult cardiomyocytes that adiponectin increased CD36 translocation and fatty acid uptake as well as insulin-stimulated glucose transport and Akt phosphorylation. Coimmunoprecipitation showed that adiponectin enhanced association of AdipoR1 with APPL1, subsequent binding of APPL1 with AMPKα2, which led to phosphorylation and inhibition of ACC and increased fatty acid oxidation. Using siRNA to effectively knockdown APPL1 in neonatal cardiomyocytes, we demonstrated an essential role for APPL1 in mediating increased fatty acid uptake and oxidation by adiponectin. Importantly, enhanced fatty acid oxidation in conjunction with AMPK and ACC phosphorylation was also observed in the isolated working heart. Despite increasing fatty acid oxidation and myocardial oxygen consumption, adiponectin increased hydraulic work and maintained cardiac efficiency. In summary, the present study documents several beneficial metabolic effects mediated by adiponectin in the heart and provides novel insight into the mechanisms behind these effects, in particular the importance of APPL1.
Keywords: AMP-activated protein kinase, fatty acid, metabolism
there is currently great interest in elucidating the mechanisms by which obesity can influence myocardial remodeling (2). Changes in myocardial energy metabolism are one of the earliest measurable abnormalities in the hearts of obese animals or humans and precedes measurable changes in in vivo cardiac function (1, 7, 16, 31, 32, 41). Shifts in myocardial substrate utilization in obesity and diabetes are typically characterized by an increase in fatty acids (FA) utilization and a decrease in glucose utilization (34). Multiple mechanisms account for these changes in metabolism and include altered glucose transport (42), increased delivery of FA, and activation of PPARα-mediated signaling pathways (2). A well controlled balance of FA uptake and oxidation is essential in maintaining both ATP production and cardiac contractile function and may also prevent potential adverse effects associated with lipotoxicity. For example, elevated FA uptake that is not matched by a proportionate increase in FA oxidation may contribute to the accumulation of intracellular triglycerides and lipotoxic products such as ceramide, diacylglycerol, and fatty acyl-CoA, which have widespread detrimental cellular consequences (38).
Obese models such as Zucker rats exhibit a decreased ability to increase FA oxidative capacity in response to increasing FA delivery, and this has been suggested to contribute to accumulation of myocardial triglycerides and lipotoxicity (35, 46). Although, ob/ob and db/db mice have increased capacity to oxidize FA in response to increasing delivery of FA substrates, which exceeds that of wild-type hearts, these animals also exhibit evidence of lipid accumulation and lipotoxicity, mitochondrial uncoupling, and decreased cardiac efficiency (6, 7, 26). Recent studies in obese humans have yielded results that mirror the changes described in mice. In a study of severely obese females, obesity was associated with increased rates of FA oxidation, increased myocardial oxygen consumption (MV̇o2), and reduced cardiac efficiency (32).
Adiponectin has now been extensively documented to mediate several cardioprotective effects (2, 28), many of which appear to be mediated via AMPK (21, 36, 37). Although the role of adiponectin in modulating carbohydrate and lipid metabolism has been extensively studied in muscle and liver (4, 15, 39, 44, 45), only a few studies to date have investigated direct metabolic effects of adiponectin on cardiomyocyte metabolism (13, 21, 27, 29, 33). Because obesity and insulin resistance are associated with hypoadiponectinemia, the present study tested the hypothesis that adiponectin exerts direct effects on cardiomyocyte FA metabolism, which could potentially influence cardiac contractile function. Two adiponectin receptor isoforms, AdipoR1 and AdipoR2, have been characterized (43), which are now known to interact with APPL1 (adaptor protein-containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif) to mediate downstream signaling (10, 25). In this study, we characterized the effect of adiponectin on FA uptake and metabolism in cardiomyocytes and perfused hearts and investigated the mechanistic role of the APPL1-AMPK axis.
MATERIALS AND METHODS
Materials.
2-Deoxy-d-[3H]glucose was purchased from Amersham (Quebec, Canada). The AMPK inhibitor compound C was purchased from Calbiochem (San Diego, CA). Insulin (Humulin) was obtained from Eli Lilly (Toronto, ON, Canada). Horseradish peroxidase (HRP)-linked anti-rabbit antibody, β-actin antibody, acetyl-CoA carboxylase (ACC) antibody, phosphospecific antibodies for rabbit AMPK (Thr172), ACC (Ser79), and Akt (Thr308) were purchased from Cell Signaling (Beverly, MA). AMPKα1, AMPKα2, and LKB1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). AdipoR1 and AdipoR2 antibodies were obtained from IBL (Takasaki, Japan). Full-length FLAG-tagged adiponectin, which contained an oligomeric profile similar to that found in circulation, was produced in a mammalian expression system (HEK 293 cells). We used both adiponectin-containing conditioned media and adiponectin purified, using an anti-FLAG M2 monoclonal antibody affinity column (29). Anti-APPL1 antibody was produced by immunization of rabbits as previously described (10). Oil red O and triethyl phosphate were purchased from Fluka Chemie (Buchs, Switzerland). All other chemicals were purchased from Sigma (St. Louis, MO), and cell culture components were purchased from Wisent (Quebec, Canada).
Isolation of adult and neonatal cardiomyocytes.
Adult rat cardiomyocytes were isolated from male Wistar rats (250–350g). Rats were anesthetized with ketamine (100 mg/kg ip) and hearts were rapidly excised and retrogradely perfused with Krebs-Henseleit solution (in mM): 4.8 mM KCl, 1.25 mM K2HPO4, 118 mM NaCl, 1.25 mM MgSO4, 25 mM HEPES, 10 mM glucose, 10 mM butanedione, pH 7.4, and liberase (0.18 mg/ml, Roche Applied Science) at 37°C. The heart was then minced, and cardiomyocytes were isolated by sedimentation in gradually increasing calcium concentration until a final concentration of 1 mM was achieved. The cardiomyocytes were gently resuspended in serum-free Medium 199 and plated on laminin (10 μg/ml, Sigma) -precoated plates. After an adhesion period of 2 h, cardiomyocytes were cultured in Medium 199 supplemented with 5% FBS and antibiotics (100 IU/ml penicillin + 10 mg/ml streptomycin, Multicell). Primary cultures of neonatal cardiomyocytes were isolated from the ventricles of 2- to 3-day-old Wistar rats by enzymatic digestion as described previously (29). The care and use of animals for these experiments were approved by the York University Animal Care Committe (Toronto, ON, Canada).
Measurement of metabolism in vitro and in isolated working mouse hearts.
FA uptake and oxidation were measured as previously described (14, 45). ACC activity was assayed by measuring the incorporation of [14C]sodium bicarbonate into malonyl-CoA (29). Substrate metabolism was determined in hearts that were isolated from 6-wk-old C57BL6 mice (Jackson Laboratory, Bar Harbor, ME) by use of previously published protocols (26). The Krebs-Henseleit buffer was supplemented with 0.4 mM palmitate bound to 3% BSA and 5 mM glucose with or without adiponectin (4 μg/ml, final concentration). Hearts were perfused for 60 min, and rates of glycolysis, glucose oxidation and palmitate oxidation, MV̇o2, hydraulic work, and cardiac efficiency were determined as previously described (26). These studies were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Immunoblotting, immunohistochemistry and immunoprecipitation.
Sarcolemmal abundance of CD36 was determined by immunofluorecence using a specific anti-CD36 polyclonal antibody that recognizes the extracellular domain of CD36 essentially as previously described (30). Briefly, myocytes were cultured on coverslips and incubated with adiponectin (10 μg/ml). Myocytes were then quickly washed with PBS and incubated with anti-CD36 (H300; Santa Cruz Biotechnology, 1:200 dilution) for 60 min at 4°C. After myocytes were fixed in 3% paraformaldehyde for 3 min on ice, the fixative was neutralized by incubation in 1% glycine for 10 min. Myocytes were incubated in blocking buffer (5% goat serum + 3% BSA) for 30 min and then were incubated with Alexa fluor 488-conjugated secondary antibody (Molecular Probes, Invitrogen, 1:1,000 dilution, 4°C) for 30 min. The images (stacks of optical slices) of CD36 translocation were quantified by image J software. Cell surface CD36 content in neonatal cardiomyocytes was determined by an antibody-coupled colorimetric assay (14). Briefly, cells were grown in 24-well plates and, after treatment with adiponectin (10 μg/ml), washed with ice-cold PBS and incubated with anti-CD36 antibody (1:200 dilution) for 60 min at 4°C. Myocytes were fixed in 3% paraformaldehyde for 3 min on ice, and the fixative was neutralized by incubation in 1% glycine for 10 min. Cells were then incubated in blocking buffer (5% goat serum + 3% BSA) for 30 min and incubated with HRP-conjugated goat anti-rabbit antibody (1:1,000 dilution) for 60 min at 4°C. After a wash with ice-cold PBS, myocytes were incubated for 30 min at room temperature with 1 ml of o-phenylenediamine dihydrochloride (OPD) reagent (Sigma) per well. The reaction was stopped by adding 0.25 ml of HCl (3M), supernatant was collected, and absorbance was measured at 492 nm. To detect the translocation of APPL1, myocytes were double-immunostained with a mAb against dystrophin (Abcam) and an anti-APPL1 antibody. Representative images (optical single slice) of APPL1 translocation was selected to show the distribution of APPL1 close to the plasma membrane (indicated by dystrophin staining). Nuclear and cyotosol fractions were prepared using a nuclear/cytosol fractionation kit (BioVision, Mountain View, CA), and the level of LKB1 was then determined by Western blotting. Colocalization of LKB1 and APPL1 in primary cardiomyocytes were determined by immunoprecipitation. To prepare homogenates for Western analyses, perfused hearts were flash frozen, pulverized under liquid nitrogen and then processed as described previously (5), with slight modifications.
Determination of intracellular lipid content.
To examine the effect of adiponectin on lipid content, adult cardiomyocytes were grown on coverslips in six-well culture plates and incubated with or without adiponectin (10 μg/ml) for 24 h, and then lipid content was measured as previously described (30). Briefly, cardiomyocytes were fixed with 3.7% formaldehyde and stained with Oil red O. Imaging was performed on a laser scanning confocal miscroscope (Olympus fluoview 300). The intensity of intracellular lipid content was then analyzed with Image J software.
Measurement of FA uptake and oxidation and ACC activity.
FA uptake was measured using fluorescent palmitate as previously described (14). ACC activity was assayed in isolated adult cardiomyocytes by measuring the incorporation of [14C]sodium bicarbonate into malonyl-CoA (29).
siRNA-mediated knockdown of APPL1 in neonatal rat primary cardiomyocytes.
Several 21-nucleotide small interfering RNA (siRNA) sequences (Ambion, Austin, TX) designed to knock down rat APPL1 were tested in cardiomyocytes, which were incubated for 1 h in serum-free medium prior to transfection with siRNAs. The sequence that provided optimal efficiency was: APPL1, GCUUAGUUCUUGUCAUGCAtt; 50 nM was transfected into myocytes using the TransIT-TKO reagent (MirusBio, Madison, WI). After 24 h, the medium was replaced by serum-free medium, and cells were then treated with adiponectin. To confirm the efficiency of siRNA against APPL1, after 24 h of siRNA transfection cells were washed with PBS and lysed with 300 μl of cell lysis buffer [0.5 M Tris·HCl (pH 6.8), 10% (vol/vol) SDS, 15% (vol/vol) glycerol, 10% (vol/vol) β-mercaptoethanol, 0.2 mM PMSF, 10 μg/ml leupeptin, 1 mM pepstatin A, 0.5 mM Na3VO4, 0.2 mM E64, and bromophenol blue]. The lysates were then boiled at 65°C for 5 min, then syringed 5 times, and centrifuged at 12,000 rpm for 1 min at 4°C. Equal amounts of lysate were resolved in 8% of SDS-PAGE and immediately transferred to PVDF membranes, which were then probed overnight at 4°C with APPL1 andtibody. Bands were visualized by chemiluminescence after a 1-h incubation with HRP-conjugated secondary antibody and also probed with β-actin to control for loading. For cells with APPL1 knockdown, FA uptake and oxidation, in the presence or absence of adiponectin (1 h) were determined using [3H]palmitate and [1-14C]palmitate, as we previously described (29).
Measurement of glucose uptake and insulin signaling.
Glucose uptake was determined by measuring uptake of 2-deoxy-d-[3H]glucose as described previously (14). Briefly, adult cardiomyocytes were pretreated with adiponectin (10 μg/ml) for 15 min followed by insulin (10 nM) treatment for 5 min in the continued presence of adiponectin. After treatment, cardiomyocytes were incubated in transport solution (140 mM NaCl, 20 mM HEPES-Na, 2.5 mM MgSO4, 1 mM CaCl2, 5 mM KCl, 10 μM 2-deoxyglucose, 0.5 μCi/ml 2-deoxy-[3H]glucose, pH 7.4) for 5 min at room temperature. Cells were then lysed and transferred to scintillation vials for 3H radioactivity counting. Values were calculated as picomoles per milligram of protein per minute. Phosphorylation of Akt at Thr308 and total Akt level were determined by Western blot. Cardiomyocytes were incubated with insulin (10 nM) for 5 min with adiponectin (10 μg/ml) pretreatment for 15 min, and then lysates were prepared and analyzed as decribed before (14).
Statistical analysis.
Data are expressed as mean values ± SE and number of replicates (n) stated in each case. Statistical analysis was undertaken using one-way ANOVA with Student-Newman-Keuls post hoc analysis or the paired Student's t-test where appropriate. Differences between groups were considered statistically significant when P < 0.05.
RESULTS
Adiponectin increases CD36 translocation and FA uptake and insulin sensitivity in primary adult cardiomyocytes.
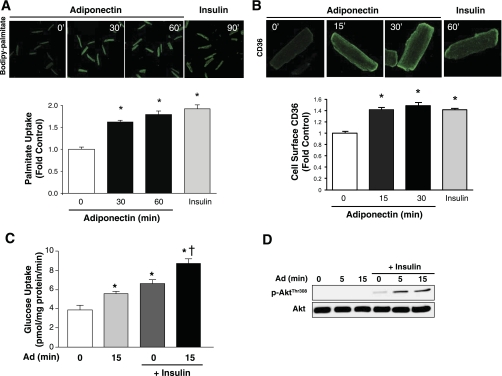
The ability of physiologically relevant levels of adiponectin (10 μg/ml) to induce long-chain FA uptake in adult rat cardiomyocytes was assessed with fluorescence-labeled palmitate. Palmitate uptake was increased following 30 and 60 min of adiponectin treatment (Fig. 1A). Insulin served as a positive control. Although similar observations were made previously in neonatal cardiomyocytes, the mechanism remained unclear. Therefore, we measured CD36 translocation and membrane insertion in intact cells with an antibody directed against an exofacial epitope of CD36. Representative fluorescent images clearly show enhanced cell surface CD36 in response to adiponectin in primary adult cardiomyocytes, and quantitative analysis of multiple experiments showed increases of 1.5-fold at 15 and 30 min, respectively (Fig. 1B). Adiponectin also increased basal and insulin stimulated glucose uptake, and increased the ability of insulin to phosphorylate Akt. (Fig. 1, C and D).
Fig. 1.
Adiponectin (Ad) increases fatty acid (FA) uptake and cell surface CD36 and sensitizes insulin's actions in adult cardiomyocytes. Isolated rat cardiomyocytes were left unstimulated (0 min, Control) or stimulated with 10 μg Ad or with 100 nM insulin for the durations indicated in the figures. Representative confocal images and quantification of FA uptake (A) or cell surface CD36 (>100 cells per condition; B). C: quantification of 2-deoxyglucose uptake in cardiomyocytes stimulated with 10 nM insulin for 5 min with or without pretreatment with 10 μg Ad for 15 min. D: representative immunoblot showing that Ad sensitizes insulin stimulation of Akt phosphorylation (Thr308). Data are means ± SE of ≥4 independent experiments. *P < 0.05 vs. Control (0 min Ad treatment); †P < 0.05 vs. insulin alone.
APPL1-dependent signaling plays an essential role in regulating fatty acid metabolism in response to adiponectin.
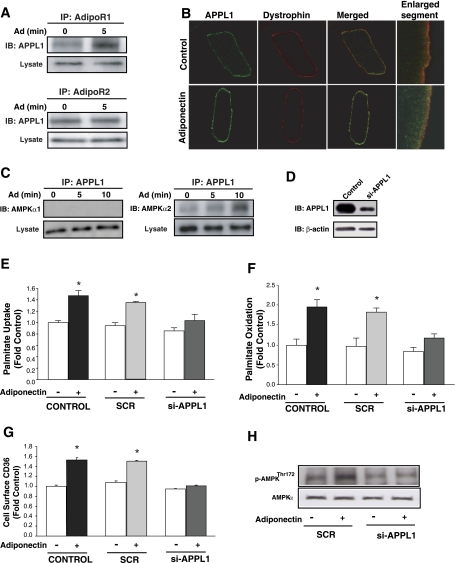
We next examined whether adiponectin increased the association of APPL1 with either of two adiponectin receptor (AdipoR) isoforms in adult cardiomyocytes. Figure 2A shows coimmunoprecipitation evidence of binding of APPL1 to both receptor isoforms under basal conditions and increased binding of APPL1 to AdipoR1 but not AdipoR2 in response to adiponectin. We also examined intracellular localization of APPL1 in adult rat primary cardiomyocytes by immunofluorescence and observed that adiponectin increased colocalization of APPL1 with the sarcolemmal marker dystrophin and increased sub-plasma membrane localization (Fig. 2B). Adiponectin also selectively increased the interaction between APPL1 and AMPKα2 but not with AMPKα1 subunit isoform (Fig. 2C). To elucidate the functional significance of APPL1-dependent adiponectin signaling, we utilized siRNA to reduce APPL1 expression in primary neonatal cardiomyocytes to ∼30% of endogenous levels (Fig. 2D), as gene silencing was not feasible in primary adult cardiomyocytes. Subsequent analysis of FA uptake (Fig. 2E), FA oxidation (Fig. 2F), and CD36 translocation (Fig. 2G) showed that APPL1 siRNA, but not scrambled siRNA, significantly attenuated the ability of adiponectin to increase FA uptake and oxidation. Importantly, adiponectin-induced stimulation of AMPK phosphorylation was also blunted by the siRNA-mediated reduction of APPL1 level in neonatal cardiomyocytes (Fig. 2H).
Fig. 2.
Functional participation of APPL1 in Ad's physiological actions in isolated rat cardiomyocytes. Representative immunoblots (IB) showing interaction between APPL1 and adiponectin receptor 1 (AdipoR1) or AdipoR2 (A) and AMPKα1 or AMPKα2 catalytic subunits (C). B: representative confocal images (optical single slice) showing individual and merged staining of cell surface APPL1 (green) or sarcolemma marker dystrophin (red). Enlarged segment is an optical single slice, magnification ×60. D: immunoblot showing knockdown of endogenous APPL1 (si-APPL1) compared with nontransfected (Control) neonatal rat cardiomyocytes. Palmitate uptake (E), palmitate oxidation (F), and CD36 translocation (G) in isolated neonatal rat cardiomyocytes not transfected (Control) or transfected with an unrelated siRNA (SCR) or siRNA against APPL1 (si-APPL1) and were treated with or without 10 μg Ad for 1 h (E and F) or 30 min (G). H: representative immunoblot showing AMPK phosphorylation (Thr172) in isolated neonatal rat cardiomyocytes transfected with SCR or si-APPL1 and subsequently treated with or without 10 μg Ad for 10 min. Data are means ± SE of ≥4 independent experiments and are expressed relative to Control (0 min). *P < 0.05 vs. Control (0 min).
Adiponectin stimulates LKB1/AMPK signaling to enhance FA uptake and oxidation.
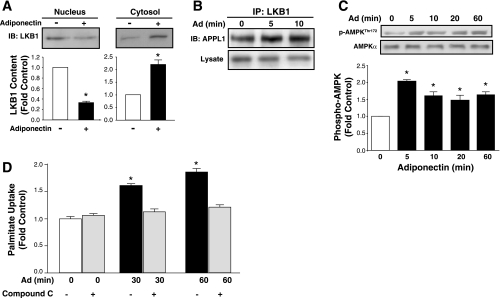
An important regulatory step in activation of AMPK by adiponectin in skeletal muscle is translocation of LKB1, an AMPK kinase, from nucleus to cytosol. Thus, we investigated the interaction between APPL1 and AMPK signaling, which has been proposed to mediate many of the metabolic effects of adiponectin. In adult cardiomyocytes, adiponectin decreased nuclear and increased cytosolic LKB1 content (Fig. 3A). Importantly, we also showed via coimmunoprecipitation that this correlated with enhanced association of LKB1 with APPL1 in response to adiponectin (Fig. 3B) and subsequent phosphorylation of AMPK (Fig. 3C). Functional consequences of this signaling axis were again analyzed, and we observed that inhibition of AMPK in adult cardiomyocytes with compound C significantly attenuated adiponectin-stimulated FA uptake (Fig. 3D). Adiponectin signaling also increased the phosphorylation of ACC (Fig. 4A), thereby inhibiting ACC activity (Fig. 4B). We ultimately confirmed the ability of adiponectin to regulate FA oxidation and demonstrated increased palmitate oxidation after 2 h (Fig. 4C). Long-term treatment of adult cardiomyocytes with adiponectin led to a decrease in cardiomyocyte lipid content determined by Oil red O staining as shown by representative image (Fig. 4D) and by quantitative analysis (Fig. 4E).
Fig. 3.
Ad activates LKB1-AMPK signaling to increase FA metabolism in adult primary rat cardiomyocytes. A: representative immunoblot and quantification of LKB1 in cytosolic and nuclear fractions in cells untreated or treated with 10 μg Ad for 5 min. B: representative immunoblot showing interaction between LKB1 and APPL1. C: representative immunoblot and quantification of AMPK phosphorylation (Thr172). D: quantification of palmitate uptake in unstimulated (Control) or stimulated with 10 μg Ad for indicated times with or without 30-min pretreatment with 10 μM compound C. Data are means ± SE of ≥4 independent experiments and are expressed relative to Control (0 min or without adiponectin). *P < 0.05 vs. Control.
Fig. 4.
Ad increases acetyl-CoA carboxylase (ACC) phosphorylation and FA oxidation and decreases ACC activity and intracellular lipid content. A: representative immunoblot and quantification of ACC phosphorylation (Ser79). B: quantification of ACC activity in adult cardiomyocytes. ACC activity determined by incorporation of [14C]sodium bicarbonate into malonyl-CoA in adult cardiomyocytes stimulated with or without 10 μg Ad for 1 or 2 h or with 1 mM 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) for 2 h. C: quantification of palmitate oxidation in cardiomyocytes untreated (0 h) or treated with 10 μg Ad for 2 or 6 h or with 1 mM AICAR for 2 h. Representative confocal images (D) and Quantification of intracellular lipid content (E) assayed using Oil red O staining in cardiomyocytes treated with 10 μg Ad or without (CON) for 20 h. Data are means ± SE of ≥4 independent experiments and are expressed relative to time-matched control (0 min). *P < 0.05 vs. Control.
Adiponectin enhances AMPK/ACC signaling, FA metabolism, and cardiac power in isolated working hearts.
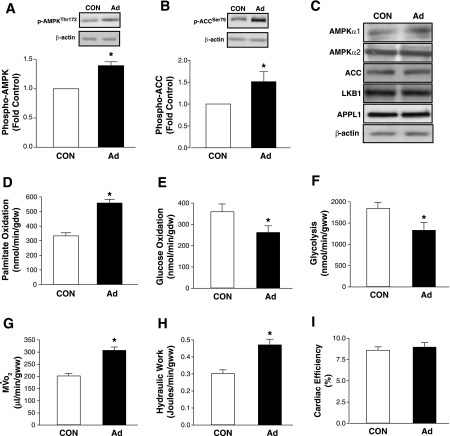
In isolated working hearts, adiponectin, immunoblot analysis of whole heart homogenates obtained from perfused hearts revealed a significant increase in the phosphorylation of AMPK and ACC (Fig. 5, A and B). Expression levels of AMPKα subunits, LKB1, APPL1, and ACC are shown and were unaltered after adiponectin perfusion (Fig. 5C). Under these conditions, adiponectin significantly increased palmitate oxidation (67%) (Fig. 5D), whereas glucose oxidation (Fig. 5E), and glycolysis (Fig. 5F) were both decreased by ∼30%. MV̇o2 (Fig. 5G) and hydraulic work (Fig. 5H) were both increased by adiponectin so that cardiac efficiency remained unchanged (Fig. 5I).
Fig. 5.
Effects of Ad on substrate metabolism, signaling molecules, and cardiac performance in perfused working mouse hearts. Representative immunoblot and quantification of phospho-AMPKThr172 (A), phospho-ACCSer79 (B), and total AMPKα1, AMPKα2, ACC, LKB1, and APPL1 (C) in the same hearts in which metabolism was measured. Quantification of Ad's effects on palmitate oxidation (D), glucose oxidation (E), glycolysis (F), oxygen consumption (MV̇o2; G), hydraulic work (H), and cardiac efficiency (I) in perfused hearts. All parameters were measured in hearts (n = 5) isolated from 6-wk-old mice perfused with 4 μg/ml Ad or without (CON) for 60 min. Data are means ± SE from, and are expressed relative to, hearts perfused without Ad for 60 min (CON). *P < 0.05 vs. CON.
DISCUSSION
Obesity is an established risk factor for heart failure, and many studies have strived to unravel the mechanisms responsible for the cardiac remodeling characteristic of obesity (2). Accordingly, adipokines have emerged as potentially important components of the pathophysiology of heart failure. In this study, we focused on adiponectin, which has been shown to exert multiple cardioprotective actions, including antiapoptotic, antifibrotic, and antihypertrophic effects (28). Because altered cardiac metabolism is one of the earliest detectable remodeling events in the progression toward heart failure (2), we previously examined the direct effects of adiponectin on palmitate and glucose metabolism in neonatal rat cardiomyocytes (29). We now provide more detailed analyses of mechanisms underlying adiponectin's cardiometabolic effect in primary adult cardiomyocytes and isolated working mouse hearts.
Adult cardiomyocytes are more reliant on FA than neonatal cardiomyocytes and respond to adiponectin by increasing palmitate uptake and oxidation. An important role for translocation of the long-chain FA transporter CD36 in cardiac metabolism has been suggested in response to stimuli such as contraction and insulin (9, 23, 24). Our results indicate that adiponectin-stimulated FA uptake in adult rat cardiomyocytes correlates with mobilization of CD36 and was a consequence of AMPK activation. Adiponectin also regulates the metabolic fate of FAs. ACC plays an important role in cardiac FA oxidation (3, 17). We observed that adiponectin increased ACC phosphorylation, with a corresponding decrease in activity, and a sustained increase in FA oxidation. Therefore, adiponectin to a certain extent mimics the impact of exercise on myocardial FA utilization. We also demonstrate in adult cardiomyocytes that adiponectin can increase basal and insulin-stimulated glucose uptake and enhance insulin-stimulated Akt phosphorylation.
Observations made regarding the regulation of metabolism by adiponectin in primary adult cardiomyocytes were supported by studies in isolated working hearts, wherein adiponectin increased rates of myocardial FA oxidation and V̇o2. Because there was a proportionate increase in cardiac function, there was no reduction in cardiac efficiency. This contrasts with observations in animal models of obesity and diabetes in which FA oxidation and MV̇o2 are increased but cardiac function and cardiac efficiency are reduced (7, 26). The reduction in cardiac efficiency in obesity is due in part to mitochondrial uncoupling and the futile FA cycling secondary to increased expression and activity of mitochondrial and cytosolic thioesterases (8). Thus, the present study suggests that adiponectin may mediate cardioprotection by increasing the efficient oxidation of FAs. It is likely that this mechanism is mediated in part by activation of AMPK. AMPK also increases GLUT4 translocation and glycolysis (20). However, the significant increase in FA oxidation in these aerobic hearts leads to reciprocal reduction in glucose utilization via the Randle Cycle. We propose that, in contrast to the changes observed in diabetes, adiponectin promotes a shift in FA utilization that is adaptive. In the heart, lipotoxicity is associated with depressed contractile function, cardiac hypertrophy, and cardiomyocyte apoptosis (11, 12, 35, 48). Increased mitochondrial FA oxidation (as occurs in response to AMPK activation) could potentially prevent or reverse cardiac lipid accumulation. Our data therefore raise the intriguing possibility that adiponectin may play a protective role in limiting lipid accumulation by enhancing cardiac FA oxidation while maintaining normal cardiac efficiency.
We have shown previously (19) that adiponectin mediates many of its effects, including cardiomyocyte metabolism (29), through receptors AdipoR1 and AdipoR2. Until recently, the immediate downstream effector of AdipoR-mediated signaling was unknown, but studies have now identified APPL1 as a novel component of adiponectin signaling (10, 25, 40). Studies by Xu's and Dong's laboratories (10, 25) showed that APPL1 plays a vital role in adiponectin-stimulated NO production and glucose transport in endothelial cells and C2C12 skeletal muscle cells, respectively. Here, we show for the first time in primary adult rat cardiomyocytes that APPL1 regulates adiponectin signaling and FA metabolism. Adiponectin stimulated the apparent recruitment of APPL1 to a sub-plasma membrane localization, which is in keeping with binding to AdipoRs located in the plasma membrane. Indeed, our immunoprecipitation studies revealed that APPL1 interacted directly with AdipoR1 and AdipoR2 and specifically identified that adiponectin increased the interaction between APPL1 and AdipoR1, but not AdipoR2, in this cell type. A similar interaction was also found in C2C12 cells in response to adiponectin (25). To determine the functional significance of APPL1 regulation by adiponectin in stimulating FA uptake and oxidation, we used neonatal cardiac myocytes in which APPL1 expression was suppressed by siRNA. Adiponectin-stimulated FA uptake and oxidation were significantly reduced in the APPL1-suppressed neonatal cardiomyocytes compared with the scrambled siRNA-treated control cells. Adiponectin-mediated CD36 translocation was also attenuated in cardiomyocytes where APPL1 had been knocked down. These results indicate that adiponectin regulates FA uptake and metabolism via an APPL1-dependent signaling mechanism.
AMPK plays a central role in mediating many of the physiological effects of adiponectin (18, 19). AMPK is regulated by AMP and phosphorylation on Thr172. We (29) previously reported that adiponectin mediates metabolic effects in neonatal rat primary cardiomyocytes by activating AMPK. We now show that the activation of AMPK by adiponectin in adult cardiomyocytes is mediated by recruitment of LKB1 to a complex that contains AdipoR1, APPL1, and AMPKα2. LKB1 is thought to be the major upstream kinase, which activates AMPK on Thr172 (22). It has been proposed that the binding of APPL1 to AdipoR is associated with translocation of LKB1 from nucleus to cytosol, interaction with APPL1, and phosphorylation of AMPK (47). Here, we observed that under basal conditions LKB1 was located predominantly in the nucleus of adult rat primary cardiomyocytes, and the translocation of LKB1 to the cytosol and association with APPL1 were also significantly increased by adiponectin. Although we did not investigate whether adiponectin altered LKB1 binding to STRADa or MO25, Zhou et al. (47) did not observe any such changes in C2C12 cells. Taken together, our findings support the existence of a signaling axis in cardiomyocytes involving APPL1, LKB1, and AMPKα2, which mediates the increase in FA uptake and oxidation in response to adiponectin.
The effects of adiponectin on cardiac metabolism and function are presently of great interest, and our current study in both primary cardiomyocytes and isolated working hearts suggests a beneficial role for adiponectin in the regulation of cardiac metabolism. We demonstrate that adiponectin increased cardiac function by promoting efficient utilization of fatty acid, and we speculate that this effect could potentially have therapeutic utility in limiting or reversing lipotoxicity in cardiac myocytes. We also provide important mechanistic insights by identifying APPL1 as an important mediator of adiponectin's direct metabolic effects.
GRANTS
This work was supported by a grant from the Heart and Stroke Foundation of Canada to G. Sweeney. X. Fang was supported by doctoral studentship awards from the Heart and Stroke Foundation of Canada and the Canadian Diabetes Association and R. Palanivel by a postdoctoral fellowship from the Heart and Stroke Foundation of Canada. K. Schram was supported by a Frederick Banting and Charles Best Canadian Graduate Scholarship from CIHR. G. Sweeney also acknowledges support from the Canadian Institutes of Health Research via a New Investigator Award. E. D. Abel is supported by National Heart, Lung, and Blood Institute Grants RO1 HL-73167 and UO1 HL-087947.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52: 434–441, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belke DD, Wang LC, Lopaschuk GD. Acetyl-CoA carboxylase control of fatty acid oxidation in hearts from hibernating Richardson's ground squirrels. Biochim Biophys Acta 1391: 25–36, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112: 2686–2695, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 114: 195–210, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol Endocrinol Metab 287: E781–E789, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107: 813–822, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144: 3483–3490, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol 43: 73–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Palanivel R, Zhou X, Liu Y, Xu A, Wang Y, Sweeney G. Hyperglycemia- and hyperinsulinemia-induced alteration of adiponectin receptor expression and adiponectin effects in L6 myoblasts. J Mol Endocrinol 35: 465–476, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, Van Arsdall M, Adrogue JV, Brown KK, Taegtmeyer H. Activation of PPARγ enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab 289: E328–E336, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Goodwin GW, Taegtmeyer H. Regulation of fatty acid oxidation of the heart by MCD and ACC during contractile stimulation. Am J Physiol Endocrinol Metab 277: E772–E777, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582: 74–80, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Li J, Hu X, Selvakumar P, Russell RR, 3rd, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab 287: E834–E841, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 67: 705–713, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23: 833–843, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52: 1627–1634, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51: 3113–3119, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mazumder PK, O'Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53: 2366–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Onay-Besikci A, Altarejos JY, Lopaschuk GD. gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem 279: 44320–44326, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med 16: 141–146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palanivel R, Fang X, Park M, Eguchi M, Pallan S, De Girolamo S, Liu Y, Wang Y, Xu A, Sweeney G. Globular and full-length forms of adiponectin mediate specific changes in glucose and fatty acid uptake and metabolism in cardiomyocytes. Cardiovasc Res 75: 148–157, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett 579: 5049–5054, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 579: 5163–5169, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Severson DL. Diabetic cardiomyopathy: recent evidence from mouse models of type 1 and type 2 diabetes. Can J Physiol Pharmacol 82: 813–823, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18: 1692–1700, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10: 1384–1389, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99: 16309–16313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 282: 7991–7996, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Lloyd SG, Zeng H, Bonen A, Chatham JC. Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 288: H2102–H2110, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562–2570, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 51: 2587–2595, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. Adiponectin activates AMPK in muscle cells via APPL1/LKB1- and PLC/Ca2+/CaMKK-dependent pathways. J Biol Chem 284: 22426–22435: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]