Abstract
The present study was designed to determine whether hyperoxia would lower the hypocapnic apneic threshold (AT) during non-rapid eye movement (NREM) sleep. Nasal noninvasive mechanical ventilation was used to induce hypocapnia and subsequent central apnea in healthy subjects during stable NREM sleep. Mechanical ventilation trials were conducted under normoxic (room air) and hyperoxic conditions (inspired Po2 > 250 Torr) in a random order. The CO2 reserve was defined as the minimal change in end-tidal Pco2 (PetCO2) between eupnea and hypocapnic central apnea. The PetCO2 of the apnea closest to eupnea was designated as the AT. The hypocapnic ventilatory response was calculated as the change in ventilation below eupnea for a given change in PetCO2. In nine participants, compared with room air, exposure to hyperoxia was associated with a significant decrease in eupneic PetCO2 (37.5 ± 0.6 vs. 41.1 ± 0.6 Torr, P = 0.001), widening of the CO2 reserve (−3.8 ± 0.8 vs. −2.0 ± 0.3 Torr, P = 0.03), and a subsequent decline in AT (33.3 ± 1.2 vs. 39.0 ± 0.7 Torr; P = 001). The hypocapnic ventilatory response was also decreased with hyperoxia. In conclusion, 1) hyperoxia was associated with a decreased AT and an increase in the magnitude of hypocapnia required for the development of central apnea. 2) Thus hyperoxia may mitigate the effects of hypocapnia on ventilatory motor output by lowering the hypocapnic ventilatory response and lowering the resting eupneic PetCO2, thereby decreasing plant gain.
Keywords: oxygen, hypocapnic ventilatory response
hypoxia promotes breathing instability during sleep by increasing the susceptibility to develop hypocapnic central apnea and periodic breathing (7, 44, 46). Similarly, episodic hypoxia increases the propensity to develop central apnea, even after the removal of the hypoxic stimulus (7). Conversely, supplemental oxygen, used as a pharmacological agent, may stabilize respiration and has been shown to reduce the frequency of central apnea in patients with heart failure (33, 39). Acute hyperoxia decreased the number of apneas and percent apnea time, although the result was inconsistent (25). The effect of hyperoxia on ventilation is likely biphasic, manifesting as initial hypoventilation (12), secondary to the inhibition of peripheral chemoreceptors, followed by gradual hyperpnea (4, 5, 20, 35, 40, 42).
Brief hyperoxic exposure (Hyp) may also promote breathing stability during sleep. Xie et al. (46) demonstrated that brief Hyp was associated with reduced hypocapnic ventilatory response, owing to inhibition of peripheral chemosensitivity. Likewise, Wellman et al. (43) demonstrated that supplemental oxygen therapy reduced “loop” gain in patients with obstructive sleep apnea with high-loop gain, but not in those with low-loop gain. The mechanisms underlying the stabilizing effect of hyperoxia on ventilatory control during sleep remain uncertain. Therefore, the purpose of our study was to determine the physiological effect of sustained hyperoxia on the hypocapnic apneic threshold (AT) during non-rapid eye movement (NREM) sleep. We hypothesized that hyperoxia lowers the AT during NREM sleep in healthy individuals by increasing the carbon dioxide reserve.
METHODS
Participants
The Human Investigation Committees of Wayne State University School of Medicine and Detroit Veterans Affairs Medical Center approved the experimental protocols, and informed, written consent was obtained from all participants. All participants were nonsmoker healthy participants, nonsnorers, free of daytime sleepiness, and free from cardiovascular, pulmonary, neurological, or other medical disorders.
Breathing Circuit
Each participant was connected to the breathing circuit via a nasal mask. An appropriate-sized, airtight silicone nasal mask (Respironics, Murrysville, PA) was glued to the face to prevent mask leaks. The mask was connected to a Plateau Exhalation Valve (Respironics, Pittsburgh, PA), via a heated pneumotachometer. The valve, which provides a continuous leak path in the breathing circuit and serves as an exhaust vent, was connected to the inspiratory line. Participants were restricted to nasal breathing by placing tape over the mouth. During the mechanical ventilation (MV), protocol hyperventilation was achieved using a pressure support ventilator [bilevel positive airway pressure (PAP) machine; Resmed Sullivan VPAP II ST-A] for which the minimum allowable continuous PAP was 2 cmH2O. Gases were introduced from external sources connected to the inspiratory line. During the Hyp, participants breathed inspired O2 fraction (FiO2) of >35% from a 100% FiO2 gas source connected to the inspiratory line to keep inspired Po2 (PiO2) ≥ 250 Torr, whereas, during sham exposure (sham), participants breathed only room air introduced into the inspiratory line from a separate source.
Measurements
Electroencephalograms, electrooculograms, and chin electromyograms were recorded using the International 10–20 system of electrode placement (electroencephalogram: C3-A2 and C4-A1; electrooculogram, O-A2). Inspiratory airflow was measured by a heated pneumotachometer (model 3700A, Hans Rudolph, Kansas City, MO) attached to a RSS 100 HR Research Pneumotach System. The tidal volume (Vt) was obtained from the electronic integration of the flow signal on the pneumotach system. To confirm the central etiology of apnea and to ascertain upper airway mechanics, supraglottic pressure was measured using a pressure transducer tipped catheter (model TC-500XG, Millar Instruments, Houston, TX), with the tip positioned in the hypopharynx. The hypopharyngeal position was obtained by advancing the catheter tip for 2 cm after it disappeared behind the tongue. End-tidal Pco2 (PetCO2) readings were obtained continuously by an infrared analyzer (model CD-3A, AEI Technologies, Pittsburgh, PA) from tubing placed in the nares via a port in the nasal mask. Arterial O2 saturation (SaO2) was measured by a pulse oximeter (Biox 3700, Ohmeda). PiO2 was sampled continuously by an infrared analyzer (model CD-3A, AEI Technologies) via tubing attached to a port on the nasal mask. The signals were displayed on a polygraph recorder (Grass model 15, Astro-Med, West Warwick, RI) and recorded using Powerlab data-acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
Experimental Protocol
Overview.
Hyperventilation via nasal MV was induced under conditions of Hyp or sham in random order in nine participants. Simple randomization was performed using a random table; the participants were blinded to the order of condition. Study participants were instructed to limit total sleep time to a maximum of 5 h on the night preceding the study. Two participants who did not maintain stable sleep on the first night repeated their respective experimental studies with zolpidem 5 mg before the study, to allow interrupted sleep. Participants assumed the supine position for the entire experimental protocol that was conducted during stable NREM sleep; hence, all trials were conducted while they were in stable stage 2 or stage 3 sleep. During Hyp, MV was conducted with continuous flow of oxygen, while the PiO2 was continuously monitored to keep PiO2 ≥ 250 Torr (see above). During sham, MV was conducted under room-air conditions with bias flow of room air. One additional participant also underwent sustained hyperoxia exposure, but could not complete the MV protocol due to inability to maintain sleep during MV. Thus 10 participants were included in the “Sustained Hyperoxia” analysis, while 9 were included in the “MV Protocol” analysis.
MV protocol.
We used nasal noninvasive positive pressure MV to produce hyperventilation to induce hyperventilation. This methodology has been previously described (7, 31, 32, 47). MV was applied for 3 min in the spontaneous-timed mode. In this mode, a backup respiratory rate is preset; timed breaths are delivered, if the participant's rate falls below the backup rate (5–8 breaths/min). The ventilator respiratory rate was set at 5–8 breaths/min, which was below the participant's eupneic rate, to prevent neuromechanical inhibition of ventilatory motor output (24). During MV, the inspiratory PAP was increased gradually in 1- to 2-cmH2O increments, starting from 2 cmH2O at the beginning of each MV trial, while keeping expiratory PAP fixed at 2 cmH2O throughout MV. MV was terminated after 3 min by returning the inspiratory PAP to the baseline expiratory PAP of 2 cmH2O. The ensuing hypocapnia resulted in either a hypopnea or central apnea. If an apnea was not induced, hyperventilation trials were repeated until apnea was induced. Central apnea was defined as an expiratory time ≥5 s. This methodology has been previously described (7, 31, 32, 47). A compressed polygraph segment, obtained from a participant in stage 2 sleep, before and during sustained hyperoxia, is shown in Fig. 1. The MV protocol was repeated on a second night under the alternate condition, Hyp or sham, in the nine participants.
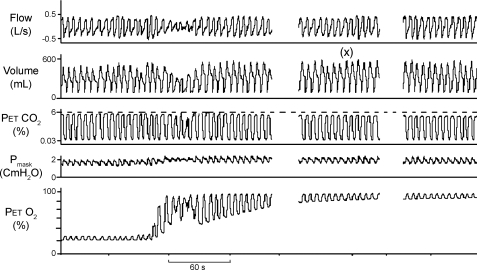
Fig. 1.
Representative compressed polygraph segments from one participant. Compressed polygraph during non-rapid eye movement (NREM) sleep shows hyperventilatory period (x) in response to sustained hyperoxia. A decline in tidal volume, at onset of hyperoxia, was not a consistent finding in all individuals. PetCO2, end-tidal Pco2; Pmask, mask pressure; PetO2, end-tidal Po2.
Data Analysis
Sustained hyperoxia before MV.
Data recorded during exposure to hyperoxia in the 10 participants before MV was used for analysis only if it was measured during stable NREM sleep. Inspired Vt, inspiration time, total breath time, breathing frequency, minute ventilation (V̇i), PetCO2, inspired end-tidal Po2, SaO2, supraglottic pressure, and upper airway resistance (RUA) were calculated breath by breath. RUA was computed using pressure-flow loops plotted for every breath; this methodology has been described previously (6, 34). For each variable, an average value was computed during the control room air period and during later hyperoxia period (sustained hyperoxia), just before the onset of MV protocol. The variables outlined above were averaged using 14 ± 4 (mean ± SD) breaths during control room air and using 5 breaths just before the onset of MV protocol (sustained hyperoxia, Table 1).
Table 1.
Results of grouped data for timing, ventilation, and resistance during room air control and sustained hyperoxia
| Variables | Control | Sustained Hyperoxia |
|---|---|---|
| Ti, s | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Te, s | 2.2 ± 0.1 | 2.0 ± 0.1 |
| fR, breaths/min | 14.6 ± 0.8 | 15.0 ± 0.8 |
| PetCO2, Torr | 41.5 ± 0.8 | 39.1 ± 0.8† |
| Vt, liter | 0.436 ± 0.03 | 0.507 ± 0.03* |
| V̇i, l/min | 6.2 ± 0.5 | 7.5 ± 0.6* |
| RUA, l·s−1·cmH2O−1 | 9.7 ± 1.1 | 7.1 ± 1.3 |
| SaO2, % | 96.5.±1.1 | 98.8 ± 0.6† |
| FiO2, % | 20.1 ± 0.8 | 74.2 ± 9.7‡ |
Values are means ± SE; n = 10 subjects (see text for details). Ti, inspiratory time; Te, expiratory time; fR, frequency of breathing; PetCO2, end-tidal Pco2 level; Vt, tidal volume; V̇i, minute ventilation; RUA, upper airway resistance; SaO2, oxygen saturation; FiO2, fraction of inspired O2.
P < 0.05,
P < 0.01, and
P < 0.001vs. control.
MV protocol.
Sleep staging (1) and scoring of arousals (38) was completed using standard criteria, analyzing trials with stable NREM sleep. We analyzed MV trials accompanied by a stable stage N2 or N3 sleep state for both Hyp and sham conditions. During the control period, five breaths recorded immediately at the onset of MV were averaged. Likewise, during the MV period, the last five mechanically ventilated breaths before the return to baseline expiratory PAP were averaged. The data analysis methodology has been previously described (7, 31, 32, 47). The AT was defined as the PetCO2 that demarcated the central apnea closest to the eupneic PetCO2. The CO2 reserve was defined as the change in PetCO2 between eupneic PetCO2 (control) and AT PetCO2 (ΔPetCO2). In addition, “hypocapnic ventilatory response” was defined as the change in V̇i between control and an apnea divided by the corresponding CO2 reserve, (ΔV̇i/ΔPetCO2 relationship), i.e., this is the slope of the ventilatory response (see Fig. 3). In one female participant, apnea could not be elicited, even at high IPAP level during both room air and hyperoxia nights; only hypopneas were recorded.
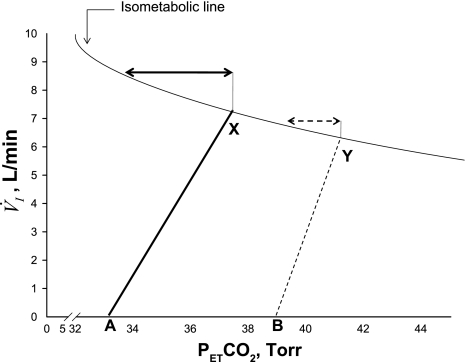
Fig. 3.
Schematic representation of the relationship between minute ventilation (V̇i) and PetCO2 along the isometabolic curve. Note decreased eupneic PetCO2 with hyperoxia (X), compared with sham exposure (Y), indicative of decreased plant gain. There is also a decrease in the slope of V̇i/CO2 with hyperoxia (solid line) compared with sham (dashed line), confirming a decline in the hypocapnic ventilatory responsiveness upon exposure to hyperoxia. The arrows indicate CO2 reserve during the two exposure conditions: greater magnitude of CO2 reserve with hyperoxia (solid arrow) compared with sham room air exposure (dashed arrow). Points A and B represent AT during hyperoxia and sham, respectively.
Statistical Analysis
Results are presented as means ± SE, unless specified otherwise. A commercially available computer statistical package was used to analyze the data (Sigma Stat 3.11.0, SPSS). The level of statistical significance was set at P < 0.05. A sample size estimation showed that to obtain a 1-mmHg change in CO2 reserve, with an estimated SD of 1 mmHg, an α-error set at 0.05 to obtain a statistical power of 80%, a sample size of 10 was required. Given that the SD obtained in our study was smaller, a sample size of nine participants who underwent MV protocol allowed adequate power.
During the baseline sustained hyperoxia period, comparisons between time points for control and hyperoxia periods were made for ventilation, timing, and RUA parameters, using paired t-test. When the normality test failed (Vt, V̇i, and SaO2), then Wilcoxon signed-rank test was used for comparison.
During MV protocol, paired t-tests were performed to compare eupneic PetCO2, AT, and CO2 reserve recorded during Hyp and sham exposures (normally distributed data). For nonparametric distributed data (hypocapnic ventilatory response), the Wilcoxon signed-rank test was used for comparison.
RESULTS
Nine participants, (4 men and 5 women) completed the MV protocol of the study, age 23.6 ± 3.8 yr, body mass index 23.6 ± 3.0 kg/m2, and neck circumference 34.2 ± 4.3 cm (means ± SD), while an additional participant completed only the baseline hyperoxia portion of the study, but was not able to maintain stable sleep during the MV protocol. Of the five women, four were studied in the follicular phase of their menstrual cycle, per reported date of menses; however, data on the fifth female participant was missing. The characteristics of the group of 10 was not different from the group that underwent MV protocol, five men and five women, age 23.4 ± 3.5 yr, body mass index 23.9 ± 3.0 kg/m2, and neck circumference 34.4 ± 4.1 cm.
Baseline Sustained Hyperoxia
Sustained hyperoxia, before onset of MV protocol, was maintained for 21.9 ± 16.7 min (mean ± SD) in 10 participants. Table 1 summarizes the results noted during control room air and sustained hyperoxia before onset of MV protocol. There was a significant increase in V̇i, with a corresponding significant drop in PetCO2 during exposure to sustained hyperoxia, before the onset of MV protocol. Thus the lower PetCO2 observed during hyperoxia was likely a result of increased V̇i upon exposure to sustained hyperoxia. A representative example of hyperventilation during sustained hyperoxia is shown in Fig. 1.
MV Protocol
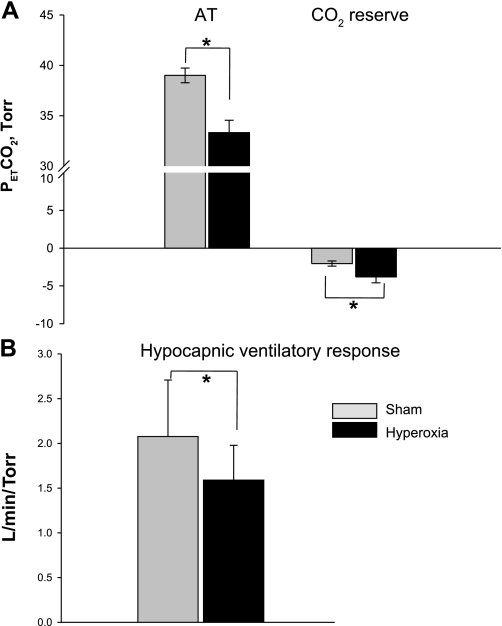
In the nine participants who completed the MV protocol, the eupneic V̇i during Hyp was significantly higher than V̇i during sham exposure (7.2 ± 0.6 vs. 5.9 ± 0.9 l/min; P < 0.05), with a correspondingly lower eupneic PetCO2 during Hyp than during sham exposure (37.6 ± 0.6 vs. 41.1 ± 0.6 Torr; P = 0.001). The CO2 reserve was significantly larger during Hyp exposure relative to sham exposure (−3.8 ± 0.8 vs. −2.0 ± 0.3 Torr; P = 0.03) (Fig. 2A). Subsequently, the AT was significantly lower during Hyp relative to the sham study (33.3 ± 1.2 vs. 39.0 ± 0.7 Torr; P = 001) (Fig. 2A). This was associated with a significant decline in the hypocapnic ventilatory response under Hyp vs. sham conditions (2.5 ± 0.5 vs. 3.7 ± 0.5 l·min−1·Torr−1; P = 0.008, Fig. 2B).
Fig. 2.
A: comparisons of apneic threshold (AT) and CO2 reserve under the two conditions, hyperoxia and room air. The major findings were that the AT was significantly lower and the CO2 reserve was significantly higher during hyperoxia vs. sham room air exposure, *P < 0.05. B: group data for hypocapnic ventilatory response under the two conditions. There was a significant decline in the hypocapnic ventilatory response during hyperoxia vs. sham room air, *P = 0.008. Solid and shaded bars represent hyperoxia and sham room air trials, respectively.
DISCUSSION
Our study demonstrated several novel findings regarding the effect of hyperoxia on the control of breathing during NREM sleep. 1) Sustained hyperoxia resulted in hyperventilation manifesting by increased V̇i and a corresponding decrease in PetCO2. 2) Sustained hyperoxia was associated with a decline in the hypocapnic ventilatory response, a widened CO2 reserve, and reduced AT.
Hyperoxic Hyperventilation
We noted that sustained hyperoxia resulted in hyperventilation, manifesting as increased V̇i and reduced PetCO2. Hyperoxia is defined as the level of Po2 inspired that produces a neural tissue Po2 greater than while breathing normobaric air (PiO2 > 160 Torr) (10, 27, 28). Multiple animal and human studies have shown hyperoxic hyperventilation, after an initial transient hypoventilation (3, 4, 5, 8, 17, 26, 42). The initial hypoventilation (15, 42), due to peripheral chemoreceptor inhibition, has not been demonstrated consistently (5, 15, 17, 20, 26, 42). Subsequent hyperventilation is likely of central origin (15, 20, 42) and manifests as increased V̇i, commencing within 5 min of hyperoxia, and may be associated with an augmented hypercapnic response (20). Conversely, recent evidence in a canine model (36) showed that there is interdependence of peripheral and central chemoreceptors, and, in fact, the gain of the central chemoreceptors seems to be very dependent on the peripheral chemoreceptor activity (36). In our study, we observed an increase in V̇i after at least 10–15 min of hyperoxia, suggesting that the central chemoreceptors have been activated. Whether the peripheral chemoreceptor had an additive, hyperadditive, or hypoadditive effect (14) in our model cannot be determined from our protocol.
There are several potential mechanisms underlying hyperoxic hyperventilation. Hyperoxia may result in increased brain tissue Pco2 via cerebral vasoconstriction (19) or the Haldane effect (22). Alternatively, hyperoxia may have a direct stimulatory effect on chemosensitive respiratory neurons (11, 27, 28, 29). For example, Mulkey et al. (28) demonstrated that hyperoxia was associated with increased production of reactive oxygen species and increased firing rate of central CO2 chemoreceptors in the solitary complex neurons of the dorsocaudal medulla oblongata. Our findings are consistent with the aforementioned studies by demonstrating the presence of hyperoxic hyperventilation in sleeping humans. The potential mechanisms of hyperoxic hyperventilation in sleeping humans are not addressed by our study.
Hyperoxia and Susceptibility to Hypocapnic Central Apnea
We noted that hyperoxia mitigated the propensity to develop central apnea during sleep. Potential contributing factors include background increased ventilatory motor output, chemoreflex sensitivity, and sleep state stability. Our findings are consistent with previous studies demonstrating a salutary effect of increased ventilatory motor output on breathing stability, as evidenced by widened CO2 reserve following central chemoreceptor stimulation with metabolic acidosis, or stimulation of the peripheral chemoreceptor with almitrine (30). Sustained hyperoxia may increase ventilatory motor output by cerebral vasoconstriction, either via direct effect of hyperoxia or a consequence of arterial hypocapnia. The net effect is reduced CO2 washout, elevated brain tissue Pco2, and increased H+ in the medulla (14).
We observed that hypocapnic chemoreflex sensitivity declined during hyperoxia, as measured by the reduced ventilatory change for a given change in PetCO2. Our findings with sustained hyperoxia are similar to previous studies demonstrating reduced CO2 sensitivity following transient Hyp exposure (9, 46). Thus hyperoxia likely blunted the peripheral responsiveness to hypocapnia, resulting in lower hypocapnic ventilatory response.
Widening of the CO2 reserve may be due to plant factors, which dampen the magnitude of hypocapnia for a given increase in ventilation. The development of hyperoxic hyperventilation and the concomitant decrease in eupneic PetCO2 also suggest reduced “plant gain” (13), as a greater change in V̇i is required for a given decrease in eupneic PetCO2 (Fig. 3, see legend).
Physiological Significance
Our findings provide a mechanistic explanation for the reported therapeutic effect of supplemental oxygen in patients with central sleep apnea, associated with high altitude (4), congestive heart failure, and idiopathic central apnea (16, 18, 33, 39). Potential mechanisms include decreased plant gain or reduced CO2 chemoreflex sensitivity (17, 25, 46). Our findings are consistent with both mechanisms, as well as increased baseline ventilatory motor output, due to increased cerebral tissue Pco2 or direct hyperoxic stimulation, producing a sustained increase in ventilation in our study. This widened the CO2 reserve by lowering the eupneic CO2, thus stabilizing respiratory control. Thus the aggregate effects of hyperoxia promoted breathing stability during NREM sleep. This may provide a therapeutic pathway for treating central sleep apnea. However, long-term safety and efficacy have not been established, given the potential for production of reactive oxygen species upon prolonged oxygen use in patients with central apnea and no evidence of hypoxemia.
Methodological Considerations
Sleep state instability may influence the AT and the hypocapnic ventilatory response; however, we analyzed trials with stable sleep state only. To achieve natural unaided sleep, we implemented moderate sleep curtailment on the night preceding the experimental protocol. Our own experience and published literature (37) demonstrate no adverse effect of moderate sleep curtailment in healthy humans on ventilatory control. In addition, study participants underwent similar sleep curtailment for both the hyperoxia and sham studies. Zolpidem was used to stabilize sleep in two individuals. It has no effect on ventilation or breathing stability and has been used by other investigators in similar studies (2, 46). Additionally, MV may induce neuromuscular inhibition if large Vt (>200% of eupneic Vt) were applied (21). Likewise, any volume effect on ventilatory control would manifest under both hyperoxia and sham nights. It is also possible that a reduction in RUA may unload the upper airway and thereby increase airflow and V̇i with reduced end-tidal CO2 levels (34) with hyperoxia; however, the effect of hyperoxia on RUA was not significant (Table 1), indicating that this was not a contributing factor to the observed findings. Additionally, Mahamed et al. (23) suggested that a decline in metabolism during sleep produces a decrease in plant gain and thereby a significant overnight reduction in chemoreflex thresholds and stable breathing in non-obstructive sleep apnea subjects. While this may have been a possibility in our study, the effect of reduced metabolism would have been a contributing factor during both hyperoxia and sham exposures and would not explain the overall decrease in plant gain noted during hyperoxia exposure alone, over and above the values noted during sham exposure. Finally, the study protocol involved delivery of a high FiO2 (PiO2 >250 Torr, FiO2 > 50%) to ensure inhibition of the peripheral chemoreceptors. This level was higher than levels (1–5 l/min) of O2 used in clinical scenarios of heart failure with central sleep apnea (16, 18), although, in one series, Franklin et al. (16) used FiO2 60% to suppress central apneas in a subset of patients. Several safety concerns are associated with high FiO2, including hypercarbia in patients with ventilatory limitation, production of reactive oxygen species, and alveolar atelectasis. Therefore, further studies are needed to determine the lowest effective dose and to ascertain long-term safety.
In conclusion, hyperoxia stabilizes breathing in healthy individuals during NREM sleep by stimulating hyperventilation, consequently widening the CO2 reserve.
GRANTS
This work was supported by the Department of Veterans Affairs and the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine, 2007 [PMC free article] [PubMed] [Google Scholar]
- 2. Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med 153: 1864–1869, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Becker H, Lambertsen CJ, Hall P, Wollman H, Goodman MW. Quantitative interactions of increased Po2 and Pco2 upon respiration in man. Ann N Y Acad Sci 190: 731–742, 1963 [DOI] [PubMed] [Google Scholar]
- 4. Becker H, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE. Ventilatory response to isocapnic hyperoxia. J Appl Physiol 78: 696–701, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Becker HF, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE. Effect of different levels of hyperoxia on breathing in healthy subjects. J Appl Physiol 81: 1683–1690, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160: 65–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol 108: 369–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cragg PA, Drysdale DB, Hamilton JH. Ventilation in intact and glossopharyngeal nerve sectioned anaesthetized rats exposed to oxygen at high pressure. J Physiol 370: 489–499, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dahan A, DeGoede J, Berkenbosch A, Olievier IC. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol 428: 485–499, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dean JB, Mulkey DK, Garcia AJ, III, Putnam RW, Henderson RA., III Neuronal sensitivity to hyperoxia, hypercapnia, and inert gases at hyperbaric pressures. J Appl Physiol 95: 883–909, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dean JB, Mulkey DK, Henderson RA, 3rd, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol 96: 784–791, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Dejours P, Labrousse Y, Raynaud Y, Teillac A. [Oxygen chemoreflex stimulus in ventilation at low altitude in man. I. At rest.] J Physiol (Paris) 49: 115–120, 1957 [PubMed] [Google Scholar]
- 13. Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 1: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dripps RD, Comroe JH., Jr The effect of the inhalation of high and low oxygen concentration on respiration, pulse rate, ballistocardiogram and arterial oxygen saturation (oximeter) of normal individuals. J Physiol 149: 277–291, 1947 [DOI] [PubMed] [Google Scholar]
- 16. Franklin KA, Eriksson P, Sahlin C, Lundgren R. Reversal of central sleep apnea with oxygen. Chest 111: 163–169, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Gautier H, Bonora M, Gaudy JH. Ventilatory response of the conscious or anesthetized cat to oxygen breathing. Respir Physiol 65: 181–196, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Gold AR, Schwartz AR, Bleecker ER, Smith PL. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis 134: 925–929, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and oxygen consumption of normal young men. J Clin Invest 27: 484–486, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lahiri S, Mulligan E, Andronikou S, Shirahata M, Mokashi A. Carotid body chemosensory function in prolonged normobaric hyperoxia in the cat. J Appl Physiol 62: 1924–1931, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Leevers AM, Simon PM, Dempsey JA. Apnea after normocapnic mechanical ventilation during NREM sleep. J Appl Physiol 77: 2079–2085, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Loeppky JA, Luft UC, Fletcher ER. Quantitative description of whole blood CO2 dissociation curve and Haldane effect. Respir Physiol 51: 167–181, 1983 [DOI] [PubMed] [Google Scholar]
- 23. Mahamed S, Hanly PJ, Gabor J, Beecroft J, Duffin J. Overnight changes of chemoreflex control in obstructive sleep apnoea patients. Respir Physiol Neurobiol 146: 279–290, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Manchanda S, Leevers AM, Wilson CR, Simon PM, Skatrud JB, Dempsey JA. Frequency and volume thresholds for inhibition of inspiratory motor output during mechanical ventilation. Respir Physiol 105: 1–16, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Martin RJ, Sanders MH, Gray BA, Pennock BE. Acute and long-term ventilatory effects of hyperoxia in the adult sleep apnea syndrome. Am Rev Respir Dis 125: 175–180, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Miller MJ, Tenney SM. Hyperoxic hyperventilation in carotid deafferented cats. Respir Physiol 23: 23–30, 1975 [DOI] [PubMed] [Google Scholar]
- 27. Mulkey DK, Henderson RA, III, Olson JE, Putnam RW, Dean JB. Oxygen measurement in brain stem slices exposed to normobaric hyperoxia and hyperbaric oxygen. J Appl Physiol 90: 1887–1899, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Mulkey DK, Henderson RA, III, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+ sensitive neurons in rat brain stem slices. J Appl Physiol 95: 910–921, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Mulkey DK, Henderson RA, III, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases intracellular pH and Na+/H+ exchange and increases excitability of solitary complex (SC) neurons from rat brain slices. Am J Physiol Cell Physiol 286: C940–C951, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med 165: 1251–1260, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 29: 95–103, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. J Appl Physiol 103: 911–916, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and Cheyne-Stokes respiration. Circ J 70: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol 92: 2565–2570, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Shock NW, Soley MH. Effect of oxygen tension of inspired air on the respiratory response of normal subjects to carbon dioxide. Am J Physiol 130: 777–783, 1940 [Google Scholar]
- 36. Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010. March 4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 161: 1124–1128, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Task Force of the American Sleep Disorders Association . EEG arousals: scoring and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 174–184, 1992 [PubMed] [Google Scholar]
- 39. Toyama T, Seki R, Kasama S, Isobe N, Sakurai S, Adachi H, Hoshizaki H, Oshima S, Taniguchi K. Effectiveness of nocturnal home oxygen therapy to improve exercise capacity, cardiac function and cardiac sympathetic nerve activity in patients with chronic heart failure and central sleep apnea. Circ J 73: 299–304, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Von Euler US, Liljestrand G, Zotterman Y. The excitation mechanism of the chemoreceptors of the carotid body. Skand Arch Physiol 83: 132–152, 1939 [Google Scholar]
- 41. Watson NA, Beards SC, Altaf N, Kassner A, Jackson A. The effect of hyperoxia on cerebral blood flow: a study in healthy volunteers using magnetic resonance phase-contrast angiography. Eur J Anaesthesiol 17: 152–159, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Watt JG, Dumke PR, Comroe JH., Jr Effects of inhalation of 100 per cent and 14 per cent oxygen upon respiration of unanesthetized dogs before and after chemoreceptor denervation. Am J Physiol 138: 610–617, 1943 [Google Scholar]
- 43. Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 162: 144–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie A, Skatrud JB, Dempsey JA. Effect of hypoxia on the hypopnoeic and apnoeic threshold for CO2 in sleeping humans. J Physiol 535: 269–278, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol 100: 171–177, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol 94: 101–107, 2003 [DOI] [PubMed] [Google Scholar]