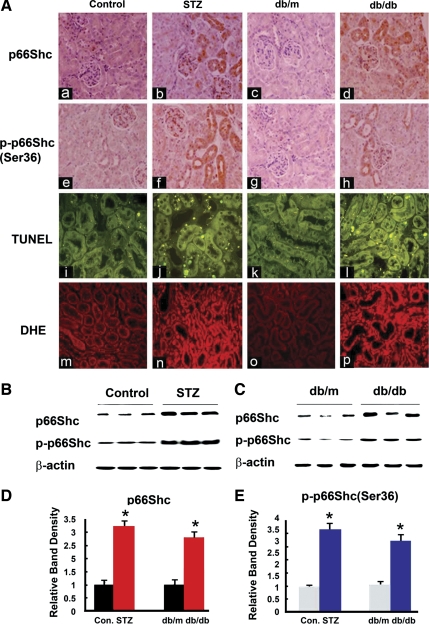
Fig. 1.
A: expression of p66Shc and phosphorylated p66Shc (at Ser36 residue) (p-p66Shc; a–h), status of apoptosis (i–l), and oxidant stress (m–p) in kidneys of control CD1 (ICR) mice (column 1: a, e, i, and m) and those with streptozotocin (STZ)-induced diabetes mice (column 2: b, f, j, and n), db/m mice (column 3: c, g, k, and o), and db/db (column 4: d, h, l, and p) mice. In control mice (ICR and db/m), p66Shc and p-p66Shc expression mainly localized in the proximal tubules, and it was increased in mice with STZ-induced diabetes and db/db mice. The terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining to mark the apoptotic nuclei also notably increased in kidney sections of diabetic mice, compared with the controls (i–l). Similarly, dihydroethidine (DHE) staining to assess the ROS generation showed increased staining in the cortical tubular cells of STZ-induced and db/db mice (m–p). Magnifications: ×200. B and C: Western blot (WB) analyses showed that both p66Shc and p-p66Shc expression were increased in the cortical kidney homogenates of diabetic mice. D and E: densitometry of the autoradiographic bands were depicted in B and C, respectively. Each bar graph represents a relative density ratio between p66Shc or p-p66Shc to β-actin. Values are means ± SE; N = 20 in each group. *P < 0.01 vs. control (Con).