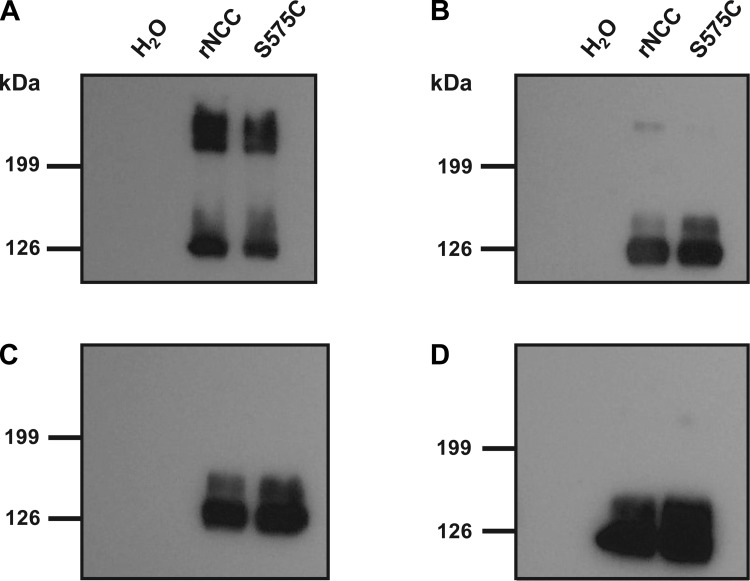
Fig. 8.
Electrophoretic behavior of rNCC and S575C-rNCC in the absence or presence of a reducing agent. Protein extracts from oocytes injected with water, rNCC cRNA, or rNCC-S575C cRNA were diluted for standard SDS-PAGE in Laemmli buffer without reducing agent (A) or containing either 0.5 (B), 2.5 (C), or 5% β-mercaptoethanol (D). In the absence of reducing agent (A), bands of expected size for monomeric and dimeric forms of the cotransporter were observed, and in both cases a diffused signal of slightly higher molecular weight was detected, which probably corresponds to glycosylated forms of the protein. This was seen for the wild-type and mutant form of rNCC. In the presence of reducing agent, the bands corresponding to the dimeric form of the cotransporter disappeared even at the lowest concentration of reducing agent used. At this concentration, we were able to observe a slight signal corresponding to the dimers, but no difference in intensity was observed between the wild type and the mutant.