Table 2.
Cyclizations with silyloxyindole substrates.a
| entry | substrate | acid chloride | major product | drb | yield(%)c |
|---|---|---|---|---|---|
| 1 | 8 | 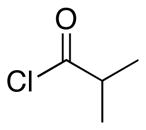 |
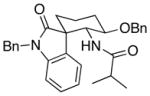 25 |
4.1:1 (2.1:1) | 68 (46) |
| 2 | 8 | 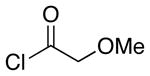 |
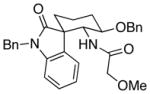 26 |
12:1 (1.7:1) | 67 (42) |
| 3 | 8 | 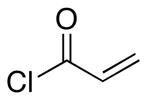 |
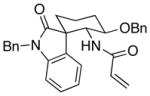 27 |
7.2:1 (3.7:1) | 38 (30) |
| 4 | 8 | 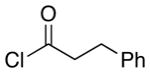 |
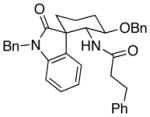 12 |
4.5:1 (2.4:1) | 66 (47) |
| 5d | 8 | 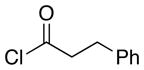 |
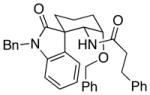 28 |
18:1 (1.8:1) | 58 (37) |
Representative procedure: Cp2Zr(H)Cl (1.25 equiv) was added to a 0.1 M solution of the indole nitrile in CH2Cl2 under Ar. After stirring at rt for 15 min the acid chloride and Sc(OTf)3 were added. The mixture stirred overnight at rt.
The ratio refers to the quaternary center. The value in parentheses refers to the ratio of the major stereoisomer to all other stereoisomers.
Combined yield of all stereoisomers. The yield of the major stereoisomer is in parentheses.
ZnCl2 was used instead of Sc(OTf)3.
