Figure 2.
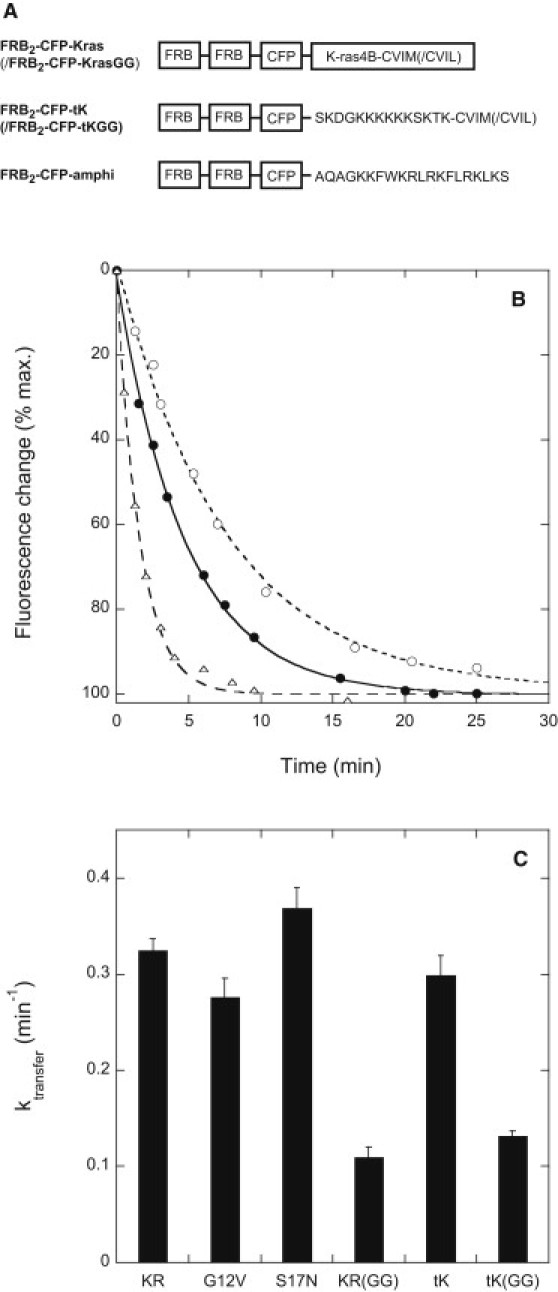
(A) Structures of the novel rapamycin-binding K-ras-incorporating protein constructs utilized in this study. CFP-Kras and CFP-Kras(GG) constructs were prepared omitting the rapamycin-binding FRB modules. (B) Representative time courses measured for rapamycin-induced relocalization of FRB2-CFP-Kras from the plasma membrane to mitochondria in HeLa cells coexpressing the mitochondrial outer membrane-anchored heterodimerization partner mitoRFP-FKBP3. Solid circles: control cells; open circles: cells treated with anti-PDEδ siRNA 24 h before plasmid transfection; open triangles: cells cotransfected with a PDEδ- and YFP-encoding bicistronic plasmid. Data were fitted using the equation x(t) = A + B exp(−ktransfert), where A, B, and ktransfer are fitting constants. (C) Rate constants ktransfer determined for rapamycin-induced redistribution of FRB-CFP-Kras and related constructs from the plasma membrane to mitochondria in HeLa cells coexpressing mitoRFP-FKBP3. Constructs are denoted as follows: tK/tKGG = FRB2-CFP-tK/-tKGG; KR/KRGG = FRB2-CFP-Kras/Kras(GG); G12V, S17N = mutant forms of FRB2-CFP-Kras. Other experimental details are as described in Materials and Methods.
