Figure 1.
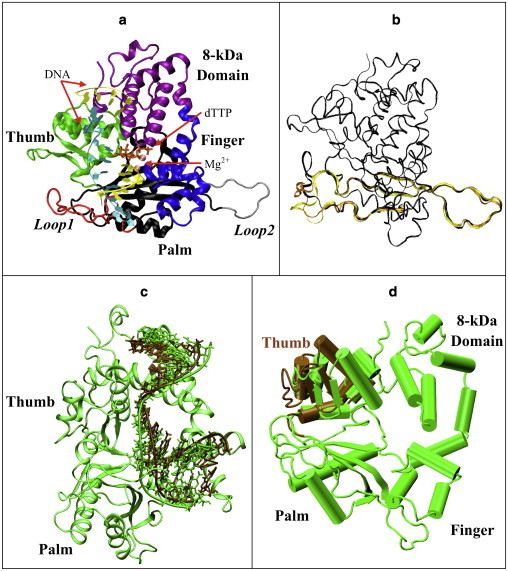
Our model of the murine pol μ ternary complex. (a) Protein domains, DNA strands, and Mg2+. (b) Starting loop conformations. Structure I (dark), from regular MD, used in simulations I, II, and VII–XVII; structure II (light), from 299 K REMD replica, used in simulations III and IV; structure III (medium), from 301 K REMD, used in simulations V and VI. Residues 360–420 of each structure are shown; other protein residues are drawn with a smaller bond radius. (c) Shifted-DNA model. DNA in the shifted-DNA model (dark) was taken from pol λ's binary structure, superimposed with DNA in pol μ's ternary structure (light). The protein is also shown as green. (d) Open-thumb model. The thumb region of pol μ (dark) was shifted to an open form comparable to pol β's binary structure and compared with pol μ's original ternary structure (light).
