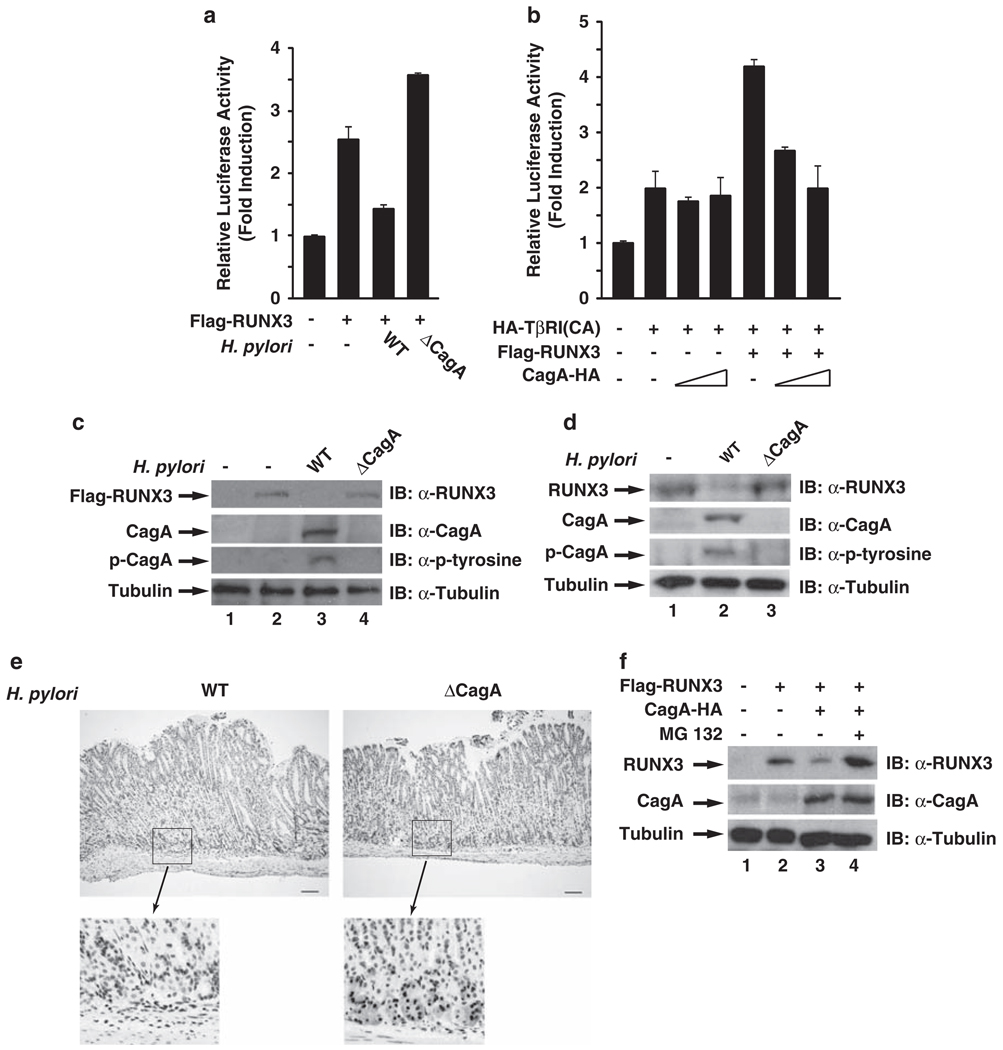
Figure 1.
H. pylori infection downregulates the expression of RUNX3 in a CagA-dependent manner. (a) CagA inhibits the transcriptional activity of RUNX3. AGS cells were transfected by Lipofectamine 2000 with TβRE reporter plasmid, together with expression vectors for RUNX3 and constitutively activated TβRI(CA) (Hanai et al., 1999; Ito et al., 2005) for 24 h. In each experiment, cells were also co-transfected with Renilla luciferase reporter plasmid as an internal control. Luciferase activity was measured 24 h after the transfected cells were infected with WT or cagA-deficient H. pylori. Results represent the average of three independent experiments ± s.d. H. pylori NCTC11637 strain and its cagA-deficient isogenic mutant were cultured as previously described (Lamb et al., 2009). H. pylori was added to AGS cells for infection at an MOI of 50–100. (b) CagA is sufficient to inhibit the transcriptional activity of RUNX3. CagA, RUNX3 and TβRI(CA) were co-transfected with the TβRE reporter plasmid into AGS cells as indicated. 48 h after transfection, luciferase activity was measured as per procedure (a). (c) CagA is essential for the reduced expression of RUNX3 in response to H. pylori infection. AGS cells transiently expressing RUNX3 were infected with WT and cagA-deficient H. pylori for 24 h, and levels of RUNX3, CagA and phosphorylated CagA were detected by immunoblotting whole-cell extracts with anti-RUNX3, anti-CagA, anti-phosphotyrosine or anti-tubulin antibodies. Antibodies against CagA, phosphotyrosine and RUNX3 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Antibodies against tubulin were purchased from Sigma (St Louis, MO, USA). (d)MKN-45 cells were infected with WT and cagA-deficient H. pylori for 48 h, and levels of RUNX3, CagA and phosphorylated CagA were detected as described in (c). (e) RUNX3 expression is decreased within mouse gastric mucosa early after infection with H. pylori NCTC11637CagA-positive strain. Immunohistochemistry for RUNX3 was performed as previously described (Ito et al., 2008) on gastric mucosa harvested from INS-GAS mice infected with WT or cagA-deficient NCTC11637 H. pylori strains 48 h after inoculation. Representative staining of RUNX3 is shown for WT (left panel) or cagA-deficient mutant (right panel)-infected mice. The bars are equal to 100 µm. Animal experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. (f) CagA is sufficient to induce the proteasome-mediated degradation of RUNX3. AGS cells were transfected with expression vectors for Flag-RUNX3 and CagA-HA. At 24 h after transfection, cells were treated or not with MG-132 (10 µm) for 6 h. Whole-cell lysates were immunoblotted for the expression of RUNX3, CagA and tubulin as indicated.