Abstract
Polymerase chain reaction analysis was performed to determine whether mosquito predators in wetland habitats feed on Anopheles gambiae sensu lato (s.l.) larvae. Aquatic mosquito predators were collected from six wetlands near Lake Victoria in Mbita, Western Kenya. This study revealed that the whole positive rate of An. gambiae s.l. from 330 predators was 54.2%. The order of positive rate was the highest in Odonata (70.2%), followed by Hemiptera (62.8%), Amphibia (41.7%), and Coleoptera (18%). This study demonstrates that the polymerase chain reaction method can determine whether aquatic mosquito predators feed on An. gambiae s.l. larvae if the predators have undigested An. gambiae s.l. in their midgut or stomach.
Keywords: Anura, aquatic insect, Coleoptera, Hemiptera, Odonata
Despite a long history of efforts to control malaria, malaria remains a major threat to human health. Because the development of a vaccine for malaria has been slow, and parasite resistance to antimalaria drugs is developing rapidly, vector control is considered the most practical method for reducing malaria transmission in developing countries (Trape et al. 2002, Fillinger et al. 2004, Killeen et al. 2004). Common measures for malaria vector control include bed nets and indoor residual spray using DDT or pyrethroids (Kouznetsov 1977, Lindblade et al. 2006). However, there is concern about toxicity to nontarget organisms (Henry and Kishimba 2006) and vector resistance to chemical insecticides (Corbel et al. 2003, Etang et al. 2003, Hargreaves et al. 2003, Casimiro et al. 2006). Because chemical insecticides also kill predators of mosquitoes (Service 1977), the reduction of predators may increase vectors; therefore, the use of chemical insecticides requires caution.
Among the various natural ecological forces controlling vector populations, predation on immature Anopheles gambiae sensu lato (s.l.) appears to be a major factor controlling population size. Predation on An. gambiae larvae contributes considerably (between 13.4 and 84.5%; Chandler and Highton 1977) to overall larval mortality (between 92.6 and 97.1%; Service 1971, 1973, 1977). However, little research has been devoted to the effects of larval predation on mosquito population structure and ecology, possibly because of difficulties in identifying and quantifying the impact of the most common predators in the field.
Using the serological method, Service (1973, 1977) found that predators of An. gambiae s.l. include amphibians as well as the insects from the orders Araneida, Odonata, Hemiptera, Hymenoptera, Diptera, and Coleoptera. Schielke et al. (2007) showed that An. gambiae s.l. DNA can be detected after ingestion by members of the families Lestidae (Odonata), Libellulidae (Odonata), and Notonectidae (Hemiptera). Although the authors fed single laboratory-raised An. gambiae sensu stricto to the predators under laboratory conditions, they recommended that the polymerase chain reaction (PCR)-based assay will be useful for future studies of An. gambiae larval ecology, particularly in detecting larval predators in natural breeding sites.
However, no studies have applied the PCR analysis to detect predators of An. gambiae larvae in natural habitats. Therefore, in the current study, we evaluated the feasibility of detecting predators of An. gambiae s.l. in natural habitats using PCR. Specifically, we were interested in whether this method can detect a variety of predators, including vertebrate predators in different types of habitats. Additionally, we examined whether time duration after collection influenced the sensitivity of PCR to detect predators.
Materials and Methods
Anopheles Abundance and Predator Collection
Our study was conducted in six wetlands (wetland A, 1 × 1 m; B, 15 × 20 m; C, 5 × 5 m; D, 2 × 2 m; E, 2 × 1 m; F, 3 × 3 m) near Lake Victoria (<≈500 m) in Mbita, Western Kenya (0°26′S, 34°12′E), on 2, 3, and 7 December 2009. This period was within the short rainy season. Water depth in wetlands ranged from 0.03 to 0.3 m. The environment of the study site has been described previously (Minakawa et al. 2008).
To estimate the density of Anopheles larvae, samples of 30 dips from the water surface were examined in each wetland using a standard dipper (diameter of dip at mouth, 11.5 cm; height, 5.5 cm; volume, 350 m). To collect mosquito predators, two field assistants swept each wetland using a 3-mm mesh D-frame dipnet (0.28 m wide) for ≈30 min. The collected specimens were immediately transferred to 99% ethanol in the field. Two hours after the first fixation, specimens were transferred to fresh 99% ethanol and were identified to genus, family, and order in the laboratory.
Dominant Dytiscidae (Laccophilus spp.) and Corixidae (Micronecta spp.) collected from wetland D were divided into two groups to examine differences in positive reaction rates at different times after collection (immediately after or 24 h after collection). The predators of the former group were transferred within 1 min to 99% ethanol in the field. The latter group was kept in a plastic container without food for 1 d (300 mm diameter; 400 mm height) filled with dechlorinated tap water to a depth of 50 mm before fixation in 99% ethanol.
PCR of Prey Found in the Midgut of Predators
PCR analysis was performed to confirm whether predators (Anuran larva, Odonata nymphs, Hemiptera, and Coleoptera) feed on Anopheles larvae. Sample An. gambiae s.l. larvae and predators were analyzed with the rRNA-encoding DNA-PCR method (Scott et al. 1993). An. gambiae s.l. larvae and predators were placed on clean paper for 30 min to remove ethanol before DNA extraction (see Ohba et al. in press). For the PCR analysis, we used the whole body of predatory insects <5 mm in body length, the abdomen of predatory insects ≥5 mm in body length, and the stomach of anuran larvae (tadpoles). DNA was extracted using REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, St. Louis, MO). The extraction solution (20 μl) and tissue preparation solution (5 μl) were mixed, and each individual sample was homogenized in a 1.5-ml tube and incubated at room temperature for 10 min, followed by incubation at 95°C for 3 min. Neutralization solution (20 μl) was added to the sample and mixed by vortexing. The resultant mixture was used directly for the PCR. Multiplex PCR was conducted using one primer for An. gambiae s.l. (Anopheles arabiensis, An. gambiae, and Anopheles merus). PCRs were performed in a total reaction mix volume of 5.0 μl that contained 0.5 μl of template DNA, 1.0 μl of REDExtract-N-Amp ReadyMix (TaKaRa Bio, Shiga, Japan), and 1 pmol of each primer. Primer sequences were as described by Scott et al. (1993): UNG, GTGTGCCCCTTCCTCGATGT; GA, CTGGTTTGGTCGGCACGTTT; ME, TGACCAACCCACTCCCTTGA; AR, AAGTGTCCTTCTCCATCCTA. The PCR mixture was heated to 95°C for 1 min and then subjected to 35 cycles of PCR amplification: 94°C for 25 s, 50°C for 25 s, and 72°C for 30 s, followed by 72°C for 5 min. The amplified DNA was loaded onto 2% agarose gel with a 100-bp ladder loading marker (Bio-Rad, Richmond, CA), stained with ethidium bromide solution (Wako, Tokyo, Japan), and visualized on an ultraviolet transilluminator (TF-20C; Vilber Lourmat, Marne-la-Vallée, France).
Statistical Analysis
To examine the effect of time duration after collection, a logistic regression analysis was applied to the data of the positive reactions in the two dominant predators, Dytiscidae (Laccophilus spp.) and Corixidae (Micronecta spp.). The response variable was binary (positive or negative reaction) for the mosquitoes ingested by predators. The time duration after collection (immediately or 24 h after collection) was used as explanatory variables. Micronecta spp. was the dominant predator in two wetlands that had different densities of Anopheles larvae (1.10 in wetland B and 0.13 in wetland C per dip; Table 1), which might have influenced the encounter rate and ease of capture by predators. Moreover, one habitat was covered with taller vegetation. We expected that the differences in habitat condition influenced the positive rates of the two populations of Micronecta spp. and that the PCR method could detect such differences. A logistic regression analysis was applied to the data from habitats B and C. The reaction (positive or negative) was used as the response variable, and wetland (B or C) was included as the explanatory variable. The significance level for all tests was set at 0.05. All statistical tests were conducted using JMP software (JMP version 8.0, SAS Institute 2008).
Table 1.
List of predators collected from the marshes and positive percentage of Anopheles gambiae s.1.
| Marsh |
A |
B |
C |
D |
E |
F |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Anopheles larvae/a dip |
4.80 |
1.10 |
0.13 |
7.10 |
0.23 |
0.13 |
||||||||
| Predators | No. tested | Positive % | No. tested | Positive % | No. tested | Positive % | No. tested | Positive % | No. tested | Positive % | No. tested | Positive % | No. tested | Positive % |
| Amphibia | ||||||||||||||
| Unidentified tadpole | 24 | 41.7 | — | — | — | — | — | — | — | — | — | — | 24 | 41.7 |
| Total | — | — | — | — | — | — | — | — | — | — | — | — | 24 | 41.7 |
| Odonata | ||||||||||||||
| Libellulidae (Pantala spp.) | — | — | — | — | — | — | — | — | 20 | 70.0 | 29 | 75.9 | 49 | 73.5 |
| Zygoptera | — | — | — | — | 8 | 50.0 | — | — | — | — | — | — | 8 | 50.0 |
| Total | — | — | — | — | — | — | — | — | — | — | — | — | 57 | 70.2 |
| Hemiptera | ||||||||||||||
| Corixidae (Micronecta spp. A) | — | — | 42 | 97.6 | — | — | 6 | 100.0 | — | — | — | — | 48 | 97.9 |
| Corixidae (Micronecta spp. B) | — | — | 9 | 88.9 | 30 | 46.7 | — | — | — | — | — | — | 39 | 56.4 |
| Corixidae (Micronecta nymphs) | — | — | 14 | 85.7 | 34 | 41.2 | — | — | — | — | — | — | 48 | 54.2 |
| Belostomatidae (Diplonychus spp.) | — | — | — | — | 20 | 5.0 | 5 | 0.0 | — | — | — | — | 25 | 24.0 |
| Notonectidae | — | — | — | — | 11 | 45.5 | 3 | 0.0 | — | — | — | — | 14 | 35.7 |
| Pleidae | — | — | — | — | 3 | 66.7 | — | — | — | — | — | — | 3 | 66.7 |
| Mesoveliidae | — | — | 2 | 100.0 | 9 | 88.9 | — | — | — | — | — | — | 11 | 90.9 |
| Total | — | — | — | — | — | — | — | — | — | — | — | — | 188 | 62.8 |
| Coleoptera | ||||||||||||||
| Dytiscidae (Laccophilus spp.) | — | — | 1 | 100.0 | 33 | 3.0 | 20 | 40.0 | — | — | — | — | 54 | 18.5 |
| Dytiscidae (species A) | — | — | 1 | 0.0 | — | — | — | — | — | — | — | — | 1 | 0 |
| Dytiscidae (species B) | — | — | — | — | 1 | 0.0 | — | — | — | — | — | — | 1 | 0 |
| Larva of Dytiscidae (unidentified) | — | — | 1 | 100.0 | 1 | 100.0 | 4 | 0.0 | — | — | — | — | 1 | 100.0 |
| Larva of Hydrophilidae (unidentified) | — | — | — | — | — | — | — | — | — | — | — | — | 4 | 0 |
| Total | — | — | — | — | — | — | — | — | — | — | — | — | 61 | 18.0 |
Results
Among the 164 An. gambiae s.l. larvae, the PCR analysis revealed that 162 were An. arabiensis (98.8%) and two were An. gambiae (1.2%). Therefore, our analysis focused on An. arabiensis prey. Over three study days, 330 aquatic predators were collected from six wetlands and killed (Table 1). The positive detection rate of An. arabiensis DNA in whole predators was 54.2% (179 of 330), which indicates that predators had recently ingested this mosquito. The predator community and the positive rates of PCR were different among the six wetlands. For instance, amphibian larvae (tadpoles) were limited to wetland A; Odonata nymphs were collected from wetlands C, E, and F; and Hemiptera and Coleoptera were collected from B, C, and D. Odonata had the highest positive rate (70.2%), followed by Hemiptera (62.8%), Anura (41.7%), and Coleoptera (18%) (Table 1). Among the Odonata, Libellulidae (Pantala spp.) had a higher positive rate than Zygoptera. Corixidae (Micronecta spp. A, B, and nymphs) and Mesoveliidae were found to have positive rates <50%, whereas Belostomatidae (Dyplonychus spp.) had the lowest positive rate of all the Hemipteran predators. The semiaquatic Hemiptera, Mesoveliidae, was found to have a higher positive rate (8 of 9). The diving beetle, Laccophilus spp. (<5 mm in body length, classified into small-bodied species; Ohba and Takagi 2010), was the dominant coleopteran predator in this study.
The results of the positive rates immediately after collection were markedly different from those 24 h after collection in two dominant predator species (logistic regression analysis: predator species, df = 1, likelihood ratio χ2 = 8.99, P = 0.003; time after collection: df = 1, likelihood ratio χ2 = 24.57, P < 0.001; Fig. 1). In both predator species, positive reactions were detected in individuals killed immediately after collection, but no positive reactions were found in those killed 24 h after collection (number of positive per number of tested, Dytiscidae: immediately after collection = 8 of 20, 24 h after collection = 0 of 20; Corixidae: immediately after collection = 6 of 6, 24 h after collection = 0 of 3).
Fig. 1.
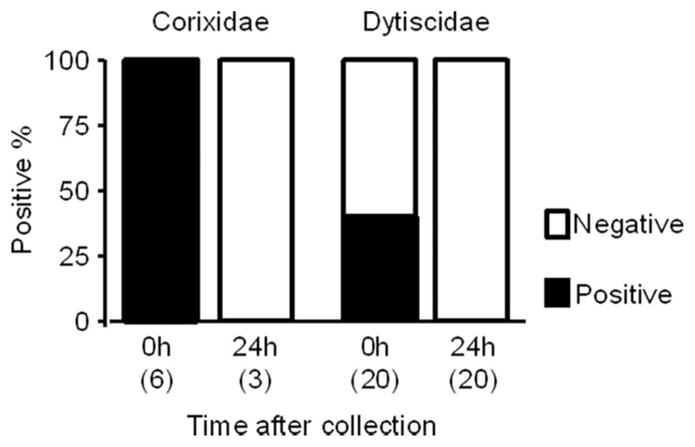
Comparison of positive rates of An. arabiensis in different time after collection of Corixidae and Dytiscidae. Numbers in parentheses show sample size.
The logistic regression analysis showed that Micronecta living in wetland B had higher positive rates of An. arabiensis in the midgut than those in wetland C (number of positive per number of tested, 20 of 23 in wetland B, 28 of 64 in wetland C; df = 1, likelihood ratio χ2 = 14.14, P < 0.001; Fig. 2).
Fig. 2.
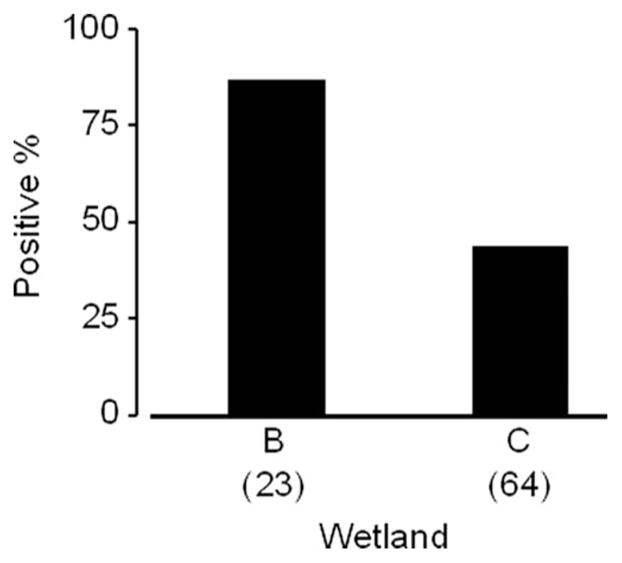
Comparison of positive rates of An. arabiensis in Micronecta spp. inhabiting in each wetland. Numbers in parentheses show sample size.
Discussion
As previously confirmed using serological methods (Service 1973, 1977), our study found that the PCR method could detect the presence of An. gambiae s.l. in the guts of predators. There were no positive reactions obtained from Dytiscidae and Corixidae predators 24 h after collection, which suggests that their stomach contents were completely digested. Hence, the PCR method was only useful when predators had undigested An. gambiae in their midgut (<24 h). Schielke et al. (2007) found that molecular assay can successfully detect larval DNA immediately after ingestion in Hemiptera and Odonata DNA extracts, but prey DNA detection varied over time and for different predators, ranging from 4 to 24 h after ingestion. In the current study, the positive rate in Odonata was higher than that in Hemiptera (Table 1). This variation in DNA prey detection appears to be related to the predator digestion type (external versus internal), which in turn affects the amount and quality of prey DNA (Schielke et al. 2007). Schielke et al. (2007) obtained a positive result 1 h after ingestion in Lestidae (Odonata), but did not detect mosquito DNA in Notonectidae (Hemiptera). The authors propose that this may be the result of Hemiptera feeding behavior; they inject digestive enzymes into their prey, ingest the liquefied material, and discard the rest of the body. This reduces the amount and quality of larval DNA in Hemiptera compared with Odonata, which ingest the entire larva.
Although only Micronecta spp. was investigated, the positive rates of An. arabiensis in the midgut of the population in wetland B were higher than those in wetland C (Fig. 2). However, the number of An. gambiae s.l. in wetland C was lower than that in wetland B, whereas the vegetation density in wetland C was higher than that in wetland B. Therefore, the difference in positive rates between the two populations of Micronecta spp. may be the result of different environmental complexity in the habitats and encounter rates of An. gambiae s.l.. The role of environmental complexity in the outcome of aquatic predation requires further study. Regardless, our results suggest that the PCR method can be used for comparing predators from different types of habitats.
Much attention has been given to identifying various predators of mosquitoes (for a review, see Mogi 2007, Quiroz-Martinez and Rodriguez-Castro 2007). Jenkins (1964) compiled a list of 220 species of invertebrate predators of mosquitoes; however, only a few of these are potential candidates for biological control. Of these species, aquatic Hemiptera (Notonectidae, Belostomatidae, Nepidae, and Naucoridae), which inhabit rice fields and wetlands, are ecologically important mosquito predators (Mogi 2007, Quiroz-Martinez and Rodriguez-Castro 2007). In the current study, >60% of aquatic Hemiptera showed a positive detection rate for An. arabiensis DNA (Table 1). Although it has been suggested that Belostomatidae are important mosquito predators (Saha et al. 2007, 2010), this family was found to have the lowest positive rates of the Hemiptera included in our study. A previous serological study (Service 1977) also showed that the positive rate in Belostomatidae (genus Sphaerodema) was lower than for other Hemipteran species. Assuming that the digestion rates of prey are similar among aquatic Hemipterans, the Belostomatidae might not be important predators of An. gambiae s.l. However, the positive rate of the semiaquatic Mesoveliidae in our study was >90% (Table 1). Because Mesoveliidae inhabit the water surface (e.g., Chen et al. 2005), they directly attack An. gambiae s.l. larvae just under the water surface.
The positive rates of Dytiscidae were considerably lower compared with the other taxa (Table 1). Beetles captured in the current study were <9 mm in body length and were classified as a small-bodied species. According to Ohba and Takagi (2010), predation rates are higher in medium-bodied species (9–20 mm in body length) than in small (<9 mm)- and large-bodied (>20 mm) species. In a preliminary field survey in May 2009, the density of medium-bodied predatory diving beetles (Coleoptera, Dytiscidae) in wetland habitats was higher during the high rainy season than during the low rainy season (S.-Y.O., unpublished data). Given these preliminary findings, it would be interesting to collect Dytiscidae during the rainy season to determine their positive rates and examine their role as predators of mosquito larvae.
This study showed that the PCR method is useful for detecting a variety of predators for malaria vectors in natural aquatic habitats. Future studies can apply the PCR method to terrestrial predators such as spiders, assassin bugs, and shorefly (Service 1971, 1973, 1977, Minakawa et al. 2007, Futami et al. 2008) to determine whether these organisms prey upon adults in the An. gambiae complex.
Acknowledgments
We thank Emiko Kawashima and Yuri Sonoda of Nagasaki University and the International Center of Insect Physiology and Ecology (ICIPE) staff for assisting us with this study. This work was supported in part by the Global COE Program, Nagasaki University, and the joint research fund between Nagasaki University and Sumitomo Chemical (Osaka, Japan).
References Cited
- Casimiro S, Coleman M, Hemingway J, Sharp B. Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. J Med Entomol. 2006;43:276–282. doi: 10.1603/0022-2585(2006)043[0276:iriaaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chandler JA, Highton RB. Breeding of Anopheles gambiae Giles (Diptera: Culicidae) in rice fields in the Kisumu area of Kenya. J Med Entomol. 1977;13:211–215. doi: 10.1093/jmedent/13.2.211. [DOI] [PubMed] [Google Scholar]
- Chen PP, Nieser N, Zettel H. The aquatic and semi-aquatic bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia. Brill Academic Publishers; Leiden, The Netherlands: 2005. [Google Scholar]
- Corbel V, Hougard JM, N’Guessan R, Chandre F. Evidence for selection of insecticide resistance due to insensitive acetylcholinesterase by carbamate-treated nets in Anopheles gambiae sensu stricto (Diptera: Culicidae) from Cote d’Ivoire. J Med Entomol. 2003;40:985–988. doi: 10.1603/0022-2585-40.6.985. [DOI] [PubMed] [Google Scholar]
- Etang J, Manga L, Chandre F, Guillet P, Fondjo E, Mimpfoundi R, Toto JC, Fontenille D. Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera: Culicidae) in the Republic of Cameroon. J Med Entomol. 2003;40:491–497. doi: 10.1603/0022-2585-40.4.491. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Sonye G, Killeen GF, Knols BG, Becker N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Trop Med Int Health. 2004;9:1274–1289. doi: 10.1111/j.1365-3156.2004.01335.x. [DOI] [PubMed] [Google Scholar]
- Futami K, Sonye G, Akweywa P, Kaneko S, Minakawa N. Diving behavior in Anopheles gambiae (Diptera: Culicidae): avoidance of a predacious wolf spider (Araneae: Lycosidae) in relation to life stage and water depth. J Med Entomol. 2008;45:1050–1056. doi: 10.1603/0022-2585(2008)45[1050:dbiagd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17:417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- Henry L, Kishimba MA. Pesticide residues in Nile tilapia (Oreochromis niloticus) and Nile perch (Lates niloticus) from Southern Lake Victoria, Tanzania. Environ Pollut. 2006;140:348–354. doi: 10.1016/j.envpol.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Jenkins DW. Pathogens, parasites and predators of medically important arthropods. Bull WHO. 1964;30:1–150. [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Seyoum A, Knols BG. Rationalizing historical successes of malaria control in Africa in terms of mosquito resource availability management. Am J Trop Med Hyg. 2004;71(Suppl. 2):87–93. [PubMed] [Google Scholar]
- Kouznetsov RL. Malaria control by application of indoor spraying of residual insecticides in tropical Africa and its impact on community health. Trop Doct. 1977;7:81–91. doi: 10.1177/004947557700700216. [DOI] [PubMed] [Google Scholar]
- Lindblade KA, Gimnig JE, Kamau L, Hawley WA, Odhiambo F, Olang G, Ter Kuile FO, Vulule JM, Slutsker L. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. J Med Entomol. 2006;43:428–432. doi: 10.1603/0022-2585(2006)043[0428:iosuoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Futami K, Sonye G, Akweywa P, Kaneko S. Predatory capacity of a shorefly, Ochthera chalybescens, on malaria vectors. Malar J. 2007;6:104. doi: 10.1186/1475-2875-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa N, Sonye G, Dida OG, Futami K, Kaneko S. Recent reduction in the water level of Lake Victoria has created more habitats for Anopheles funestus. Malar J. 2008;7:119. doi: 10.1186/1475-2875-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M. Insects and other invertebrate predators. Biorational Control of Mosquitoes. In: Floore TG, editor. J Mosq Control Assoc. Suppl. Vol. 7. 2007. pp. 93–109. [DOI] [PubMed] [Google Scholar]
- Ohba S, Takagi M. Predatory ability of adult diving beetles on the Japanese encephalitis vector Culex tritaeniorhynchus. J Am Mosq Control Assoc. 2010;26:32–36. doi: 10.2987/09-5946.1. [DOI] [PubMed] [Google Scholar]
- Ohba S, Trang Huynh TT, Kawada H, Loan Luu L, Tran Ngoc H, Le Hoang S, Higa Y, Takagi M. Heteropteran insects as mosquito predators in water jars in southern Vietnam. J Vector Ecol. doi: 10.1111/j.1948-7134.2011.00154.x. (in press) [DOI] [PubMed] [Google Scholar]
- Quiroz-Martinez H, Rodriguez-Castro A. Aquatic insects as predators of mosquito larvae. Biorational Control of Mosquitoes. In: Floore TG, editor. J Mosq Control Assoc. Suppl. Vol. 7. 2007. pp. 110–117. [DOI] [PubMed] [Google Scholar]
- Saha N, Aditya G, Bal A, Saha GK. A comparative study of predation of thee aquatic heteropteran bugs on Culex quinquefasciatus larvae. Limnology. 2007;8:73–80. [Google Scholar]
- Saha N, Aditya G, Saha GK, Hampton SE. Opportunistic foraging by heteropteran mosquito predators. Aquat Ecol. 2010;44:167–176. [Google Scholar]
- SAS Institute. JMP, version 8. SAS Institute; Cary, NC: 2008. [Google Scholar]
- Schielke E, Costantini C, Carchini G, Sagnon NF, Powell J, Caccone A. Short report: development of a molecular assay to detect predation on Anopheles gambiae complex larval stages. Am J Trop Med Hyg. 2007;77:464–466. [PubMed] [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Service MW. Studies on sampling larval populations of the Anopheles gambiae complex. Bull WHO. 1971;45:169–180. [PMC free article] [PubMed] [Google Scholar]
- Service MW. Mortalities of the larvae of the Anopheles gambiae Giles complex and detection of predators by the precipitin test. Bull Entomol Res. 1973;62:359–369. [Google Scholar]
- Service MW. Mortalities of the immature stages of species B of the Anopheles gambiae complex in Kenya: comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. J Med Entomol. 1977;13:535–545. doi: 10.1093/jmedent/13.4-5.535. [DOI] [PubMed] [Google Scholar]
- Trape JF, Pison G, Spiegel A, Enel C, Rogier C. Combating malaria in Africa. Trends Parasitol. 2002;18:224–230. doi: 10.1016/s1471-4922(02)02249-3. [DOI] [PubMed] [Google Scholar]


