Summary
Pathogens have been proposed as potentially important drivers of population dynamics, but while a few studies have investigated the impact of specific pathogens, the wealth of information provided by general indices of health has hardly been exploited. By evaluating haematological parameters in wild populations, our knowledge of the dynamics of health and infection may be better understood.
Here, haematological dynamics in natural populations of field voles are investigated to determine environmental and host factors associated with indicators of inflammatory response (counts of monocytes and neutrophils) and of condition: measures of immunological investment (lymphocyte counts) and aerobic capacity (red blood cell counts).
Individuals from three field vole populations were sampled monthly for 2 years. Comparisons with individuals kept under controlled conditions facilitated interpretation of field data. Mixed effects models were developed for each cell type to evaluate separately the effects of various factors on post-juvenile voles and mature breeding females.
There were three well-characterized ‘physiological’ seasons. The immunological investment appeared lowest in winter (lowest lymphocyte counts), but red blood cells were at their highest levels and indices of inflammatory response at their lowest. Spring was characterized by a fall in red blood cell counts and peaks in indicators of inflammatory response. During the course of summer—autumn, red blood cell counts recovered, the immunological investment increased and the indicators of inflammatory response decreased.
Poor body condition appeared to affect the inflammatory response (lower neutrophil and monocyte peaks) and the immunological investment (lower lymphocyte counts), providing evidence that the capacity to fight infection is dependent upon host condition.
Breeding early in the year was most likely in females in better condition (high lymphocyte and red blood cell counts).
All the haematological parameters were affected adversely by high population densities.
Keywords: haematology, Microtus agrestis, population dynamics, wildlife health
Introduction
Following a long period of neglect, pathogens have been suggested recently as being important drivers of host population dynamics (Tompkins & Begon 1999). Some studies have evaluated the impact of specific pathogens on, for example, demographic rates in cyclic populations (e.g. Burthe et al. 2008), but these inevitably fail to take account of the totality of pathogens infecting a population. It has also been proposed that the condition of individuals might be a key factor regulating wild populations (Lochmiller 1996). Although pertinent to both these questions, the wealth of information provided by general indices of health and infection used routinely in human and veterinary medicine has only rarely been exploited. Moreover, the few studies that have provided empirical data to address these issues (Newson & Chitty 1962; Mihok & Schwartz 1989; Huitu et al. 2007) have evaluated only a single or few indices of health, or have focused only upon a specific time of the year. By evaluating indices of health in wild populations, our knowledge of the dynamics of health and infection may be understood more clearly. Here, we attempt this in replicated, natural populations of a cyclic wildlife species, Microtus agrestis L. (the field vole), by evaluating dynamic patterns in haematological parameters.
Of the various blood cell types, red blood cells (erythrocytes, RBCs) and lymphocytes are important indicators of fitness and condition. Low concentrations of RBCs, caused by blood loss, haemolysis or decreased erythrocyte production, result mainly from deficient nourishment and infection or parasitism (Stockham & Scott 2002). The rodent RBC has a short life span (20–30 days) (Feldman et al. 2000), and anaemia is therefore relatively rapidly apparent.
Lymphocytes, the effectors of acquired immunity, proliferate in response to antigenic stimuli and have a long life span in blood, while their numbers decrease (lymphopenia) during immunosuppression by glucocorticoids or immunosuppressive infections (Feldman et al. 2000; Stockham & Scott 2002). Therefore, lymphocyte circulating levels may be useful indicators of immunological investment.
Of the remaining white blood cells (WBCs), blood concentrations of neutrophils increase rapidly as a response to cytokines released during tissue injury and bacterial infection (Tizard 2004). They are useful proxies for acute inflammatory response, as their levels return to normal soon after antigenic stimulation ceases. Monocytes are found in high concentrations in subacute and chronic inflammatory response caused by bacterial or protozoan infections (Feldman et al. 2000; Tizard 2004).
The interpretation of these haematological parameters requires caution, because a particular pattern may arise for a variety of reasons (Table 1). None the less, by taking the ecological context into account and integrating the values of different cell types, the present study analyses variation of health and infection in natural populations with respect to intrinsic (sex, age, reproductive status, body condition) and extrinsic (host density, season) factors. Particular attention is paid to the elaboration of seasonal patterns and possible explanations of these, and to the interrelationships between host densities, indicators of host condition and indicators of inflammatory response. Comparisons with data from individuals kept under controlled conditions are used to facilitate interpretation.
Table 1.
Main causes of fluctuation, and whether levels increase (↑), decrease (↓) or are unaffected (=), in the circulating levels of vertebrate blood cells (from Stockham & Scott 2002 and Feldman et al. 2000)
| Cell type | Sustained stress (glucocorticoids) |
Alarm response (epinephrine) |
Acute bacterial infections |
Chronic bacterial and protozoan infections |
Viruses and proteo-bacteria |
|---|---|---|---|---|---|
| Lymphocytes | ⇊ | ⇈ | ↓ | ↑ | ↓ = ↑1 |
| Neutrophils | ⇈ | ⇈ | ⇈ ↓ 2 | ↑ | ↓ = ↑1 |
| Monocytes | ↓ ↑ 3 | = | ↑ | ⇈ | = |
| Red blood cells* | = | ↑ 3 | ↓ | ↓ | ↓ |
⇈: Usually. ↑: In some cases.
Depending on the pathogen.
Overwhelming inflammation is often associated with a decrease of neutrophil levels, accompanied by evident clinical manifestations.
Depending on the host species.
Besides the effects of deficient nourishment and metazoan parasites.
Materials and methods
SAMPLING IN THE FIELD
Field voles were sampled every 4 weeks (primary sessions) over a 2-year period (April 2005—March 2007), apart from 8-week gaps between November and February, at four sites (BHP, KCS, PLJ and ROB) in Kielder Forest, UK (55°13′ N, 2°33′ W; Burthe et al. 2008). Distances between sites ranged from 4 to 20 km. Collection of haematological data at one of the sites (KCS) was discontinued after six sessions in order to cope with the large number of samples. At each site, a trapping grid measuring 50 × 50 m was established, with 100 Ugglan special live capture traps (Grahnab, Hillerstorp, Sweden), set at approximately 5-m intervals. In each primary session, the traps were checked five times (secondary sessions), at sunrise and before sunset (approximately 12-h intervals).
Individuals were marked uniquely and permanently on first capture with a small microchip transponder (Labtrac; AVID plc, Uckfield,UK). On first capture within a primary session, each vole was assessed for pelage (juvenile coat, first moult, adult coat), sex, body mass (to the nearest 0·5 g), body condition and reproductive status. Body condition was evaluated by estimating by palpation the degree of fat cover over the vertebral column and the pelvic bones, giving a score between 2 and 10 (Burthe et al. 2006). For this study, reproductive status was considered only for females, which were classified as ‘non-gravid’, ‘gestating’, ‘possibly gestating’ (omitted from reproductive status analysis) or ‘lactating’ (if they were both ‘lactating’ and ‘gestating’, they were considered ‘gestating’).
To minimize possible biases in the profile of WBC types due to an alarm response (Feldman et al. 2000) handling and sample collection procedures were standardized, blood being taken directly from the tail-tip into a heparin-coated capillary tube as the last procedure carried out at first capture during each primary session (as a pilot study showed no effect of handling time under these circumstances).
HAEMATOLOGICAL PARAMETERS
Blood samples were processed in a field laboratory within 8 h of collection. Coagulated samples, or those from clinically dehydrated individuals (n = 4), were discarded. To cope with very small samples, 2 μL of non-coagulated blood were obtained with a micropipette from the capillary tube and diluted 1 : 10 in 1 m phosphate-buffered saline (PBS). This was used to produce two final dilutions: 1 : 20 in 4% acetic acid with 1% crystal violet and 1 : 5000 in PBS, to count WBCs and RBCs, respectively, using Kova Glasstic® (Hycor Biomedical Ltd, Penicuik, UK) slides with grids. The number of WBCs or RBCs lying in specified squares of the grids were counted (12 squares for WBCs and four for RBC) to determine the number of cells per microlitre. The remaining blood was used to produce blood smears for differential WBC count. Smears were air-dried, fixed with methanol and stained with Rapid Romanowsky Stain Pack—HS705 (HD Supplies, Aylesbury, UK). At least 100 WBCs were counted.
HAEMATOLOGY IN CAPTIVE ANIMALS
Samples were also taken from individuals kept under controlled conditions. A colony was created in 2004 from voles brought from Kielder Forest. They were kept at 20 °C, fed commercial pelleted rodent food and allowed water ad libitum. Twelve hours of artificial light were provided, but with natural light the photoperiod ranged from 12 to 17 h; these conditions allow year-round reproduction. Soon after weaning, 24 field voles were arranged in pairs, six of the same sex (non-breeding), six consisting of one female and one male (breeding). Thereafter, a blood sample was collected every 4 weeks for approximately 1 year or until they died. To increase sample size, stock individuals were also sampled on a single occasion. The number of samples processed totalled 185.
STATISTICAL ANALYSIS
General approach
Analyses of the haematological parameters addressed four issues. First, while controlling for other factors, captive and wild field voles were compared. Secondly, the effects of intrinsic and extrinsic factors in the animal house were investigated. Two further analyses were conducted with the field data: on the effect of intrinsic and extrinsic variables on all post-juveniles, and on the effect of the reproductive status of adult females during the breeding season.
In all analyses, blood cell numbers were the response variables, treated as continuous variables because of the large numbers involved. Skewed distributions were normalized by the appropriate power transformation, as suggested by the ‘boxcox’ function in the mass library of the statistical software package r (R Foundation for Statistical Computing, http://www.r-project.org).
In general, analyses were conducted using linear mixed effects models with random intercepts (LMMs), using the function ‘lmer’ in the ‘lme4’ library of r. Because control for confounding and interaction phenomena is a key factor when analysing observational data (Kleinbaum et al. 1998), the construction of the statistical models was performed by backward elimination starting from a maximum model that included the explanatory variables of interest, plus potential confounders and effect modifiers, and the relevant pairwise interaction terms. Terms were eliminated using the Akaike information criterion (AIC) (Akaike 1974). They were removed unless they reduced the AIC by more than 2 units when included, but because a ΔAIC of 2 may select overly complex models incorrectly when the true effect of a measured factor is relatively weak, data are highly overdispersed or data are few (Richards 2008), the ΔAIC resulting from the removal of each significant term is reported and discussion focused upon those variables with higher ΔAIC values and stronger associations.
Coefficients and P-values were estimated by restricted maximum likelihood (REML) methods but, as the likelihoods of models with different fixed effects are not comparable when REML is used, maximum likelihood methods were used during the backward elimination processes. Due to unresolved issues regarding estimation of the degrees of freedom associated with fixed effect coefficients in mixed models (Bates 2006), the robustness of results was assessed using Markov Chain Monte Carlo simulations (function ‘mcmcsamp’ in r). Each model was simulated 1000 times and 95% probability confidence intervals were constructed for each coefficient.
Captive vs. wild haematology
To compare free-ranging and captive field voles, we used LMMs of the form:
where the explanatory variable of interest was captivity status and the other variables were introduced to control for confounding and effect-modification phenomena. The unique identification number given to each vole (VOLE_ID) was used as a random effect to account for correlation among observations of the same individual. ‘Breeding status’ in the captive colony was determined by the sex of the individual in the same cage. In the field, months for a particular site were classified as ‘breeding’ if at least 25% of the adult females were either gestating or lactating. Individuals with a juvenile coat and ≤ 17 g in weight were classed as juvenile. Differences between the variances of each cell type in free-ranging and captive individuals were assessed by Bartlett’s tests.
Variation in haematological parameters in the animal house
The effects of age (in days, plus age2 to correct for non-linearity), sex and breeding, plus pairwise interactions, were evaluated using LMMs. VOLE_ID was again used as a random effect. Similar models involving only breeding females were constructed that also included the variable REPR, which classified mature females as ‘non-gravid’, ‘gestating’ or ‘lactating’. As the purpose was to compare the results with those obtained with the field data, only the significant associations between blood cell concentrations and explanatory variables are reported.
Variation in haematological parameters in the field
For the field data, juveniles (see above) were not considered in our analysis because exploratory examination of the data suggested substantial changes in blood cells during the first weeks of life.
The analysis considered the following fixed effects. Age was approximated using a dichotomous variable (AGE_CLASS). ‘Adult’ and ‘young’ individuals were distinguished on the basis of capture histories as being older and younger than 90 days, respectively. In the absence of enough trapping history, non-juveniles were classified as adult if they weighed 22 g or more, and otherwise young. Once classified as an adult, individuals remained so for every successive capture. The alternative use of weight (WGT) was evaluated by AIC in the maximum models to determine if it was a better descriptor than AGE_CLASS. Body condition scores (BODYCOND) and SEX were employed in the analysis as recorded in the field. Whether individuals were recaptured (R) or newly captured (N) was included in the analysis as the R/N variable.
Alternative seasonal classifications were explored using AIC in the maximum models to determine which was optimal (data not shown). Cycles using two sinusoidal components to reflect seasonality (SEASON[sin] + SEASON[cos]) (Diggle 1990) were the best proxies of season in three of the four cell types examined, and therefore they were used for every analysis for consistency.
Population sizes were estimated using Huggins’s closed capture models within a robust design (Huggins 1989; Kendall & Nichols 1997). The analysis was conducted in program mark using mixture models (Pledger 2000) to allow heterogeneity in capture probabilities. The population densities investigated were current estimates (DENSITY-0; mean of the current and previous month’s population estimate), and densities at lags of 3 and 6 months, to cover direct and delayed density-dependence in a manner comparable to previous studies on field voles (Burthe et al. 2006).
The maximum LMMs included all the variables to be explored and all relevant two-way interactions. As voles sampled at the same site in the same month shared the same population level covariates, analyses also included the interaction between site and month as a random effect (SITE * SESSION) (Telfer et al. 2005). However, due to the relatively low number of captures per animal (mean = 2·2) it was not possible to include VOLE_ID as a random effect in the maximum model. VOLE_ID was added to the restricted model to verify that accounting for non-independence of samples from the same animal did not alter the results.
Due to the large number of variables in the maximum model, a first restriction of the model was carried out by chunkwise elimination (Kleinbaum et al. 1998) of interaction terms that reduced AIC if removed singly using likelihood ratio tests. Further elimination of single non-significant interaction terms and main effects was carried out in a stepwise manner using AIC as described above.
Impact of female breeding status on haematological parameters
The maximum models included the following fixed effects: SEASON, REPR (see above), YEAR, the densities significant in the analysis of all field data, all two-way interactions and the random effect SITE * SESSION. As in the analysis of all field data, VOLE_ID was added after model restriction.
Results
Field data consisted of 3494 observations from 1574 field voles. The descriptive statistics of haematological parameters for natural populations and the captive colony are set out in Table S1 in the online supplementary material. After a comparison of wild and captive animals, all other haematological results are organized by cell type.
CAPTIVE VS. WILD HAEMATOLOGY
RBC counts were higher in captive voles than in the wild, but the difference was greater for breeding than non-breeding animals [Tukey’s honestly significant difference (HSD) P-values < 0·001 and 0·039, coefficients = −336·64 and −202·45, respectively]. Variances in the wild and captivity were similar (P = 0·159).
Lymphocyte and monocyte counts were higher in the wild than in captivity [coefficients ± standard error (SE) = 0·075 ± 0·009 and 0·107 ± 0·012, respectively, for lymphocytes and monocytes; both P-values < 0·001]. Neutrophil counts were higher in the wild only in spring (coefficients ± SE = 0·102 ± 0·017; P < 0·001). The variances for all WBC types were greater in the natural populations (all P-values < 0·001).
RED BLOOD CELLS
In the captive colony, RBCs increased from birth to maturity and then remained stable. Males tended to have higher counts than females, but this difference was not quite significant (P = 0·053). Gestating females had lower RBC counts than lactating and non-gravid females during the first 3 months of life (main effect P = 0·026, interaction with age/age2 P = 0·020, P = 0·007), but when older, lactating and gestating females both had significantly lower RBC counts than non-gravid animals. No differences in RBC counts were observed between breeding and non-breeding males (P = 0·115).
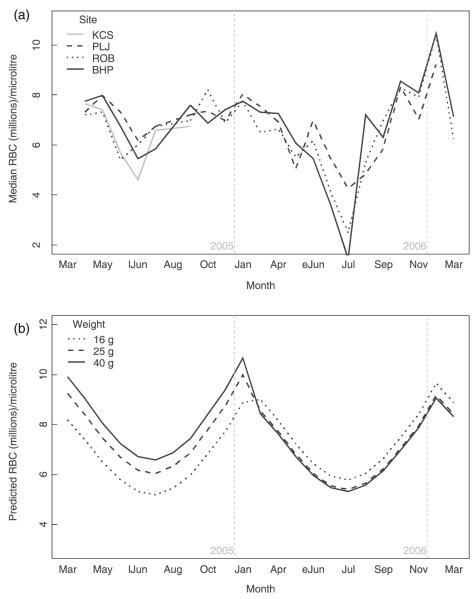
In the field, the seasonal pattern was consistent for both years at all sites, showing a sharp decline of RBC counts during spring (especially in 2006), followed by a recovery in summer, reaching maximum counts in autumn/winter (Fig. 1).
Fig. 1.
Red blood cell counts in wild field voles. (a) Observed seasonal variation of field vole red blood cells by trapping site. (b) Predicted red blood cell levels of a male field vole at a fixed past density (50): variation by month, body mass (line type) and year. (eJun = early June; lJun = late June).
In the models for the field data (Table 2), WGT was a better descriptor than AGE_CLASS. For all non-juveniles there was a positive correlation with WGT in the first sampling year, but this was reversed in the second year (Fig. 1). Males had higher counts than females. Counts were also correlated negatively with density 6 months before. Lactating and non-gravid females did not differ. However, when past density was high (more than 60 individuals in the grid), gestating voles had higher RBC counts than did lactating or non-gravid females. As high density 6 months before was seen primarily at the beginning of the breeding season (April—May), this difference occurred only during that period. As this pattern runs counter to that observed in captivity, this suggests that females with high RBC counts were the first to reproduce.
Table 2.
Mixed-effects models describing factors associated with the variation of red blood cells in all post-juvenile field voles year-round, and in adult females during the breeding season
| Term | Coefficients | SE | P-value | 95% CI for coefficients* |
ΔAIC† |
|---|---|---|---|---|---|
| Post-juveniles | |||||
| Model = RBC0·5~WGT + WGT2 + SEASON[sin] + SEASON[cos] + SEX + YEAR + DENSITY-6 + WGT*YEAR + WGT2 * YEAR Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 2715 | |||||
| Intercept | 2634·46 | 186·15 | < 0·0001 | 2260, 3041 | |
| WGT | 9·62 | 12·06 | 0·4250 | −15, 33 | - |
| WGT2 | −0·10 | 0·20 | 0·6149 | −0·49, 0·30 | - |
| SEASON[sin] | −261·28 | 41·99 | < 0·0001 | −340, −172 | 28 |
| SEASON[cos] | 237·44 | 49·16 | < 0·0001 | 146, 338 | 20 |
| SEX(male) | 97·46 | 23·88 | < 0·0001 | 54, 143 | 17 |
| YEAR(2nd) | 937·20 | 345·91 | 0·0068 | 302, 1631 | - |
| DENSITY-6 | −2·40 | 1·18 | 0·0419 | −4·72, − 0·01 | 3 |
| WGT * YEAR(2nd) | −64·41 | 24·1 | 0·0075 | −106, −12 | 5 |
| WGT2 * YEAR(2nd) | 0·87 | 0·40 | 0·0311 | 0·052, 1·58 | 3 |
| Adult females during breeding season | |||||
| Model = RBCR0·5~REPR + SEASON + YEAR + DENSITY-6 + REPR * DENSITY-6 Random effects = SITE * SESSION; the random effect VOLE_ID could not be estimated | |||||
| n = 515 | |||||
| Intercept | 3027·63 | 105·94 | < 0·0001 | 2809, 3253 | |
| REPR(gestating) | −231·92 | 104·64 | 0·0288 | −424, −25 | - |
| REPR(lactating) | −26·56 | 116·46 | 0·3912 | −271, 200 | - |
| SEASON[sin] | −350·05 | 105·82 | 0·0020 | −566, −130 | 7·7 |
| SEASON[cos] | 394·31 | 86·20 | < 0·0001 | 214, 576 | 15 |
| DENSITY-6 | −6·19 | 1·54 | 0·0003 | −9, −3 | - |
| REPR(g) * DENSITY-6 | 4·68 | 1·74 | 0·0082 | 1, 8 | 4 |
| REPR(l) * DENSITY-6 | 0·29 | 2·22 | 0·3081 | −4·1, 4·7 | 4 |
Intervals (95% probability) for the distribution of 1000 Markov Chain Monte Carlo simulations of the model.
Akaike’s information criterion (AIC) value increment if the single term is dropped.
CI: confidence interval; SE: standard error.
LYMPHOCYTES
In captivity, both males and females kept in breeding pairs experienced a sharp drop in lymphocyte counts when reproduction began (average counts went from 4120 to 3190 lymphocytes μL−1; interaction breeding × age P = 0·047), but after 90 days of age, this difference disappeared (interaction breeding × age2 P = 0·063).
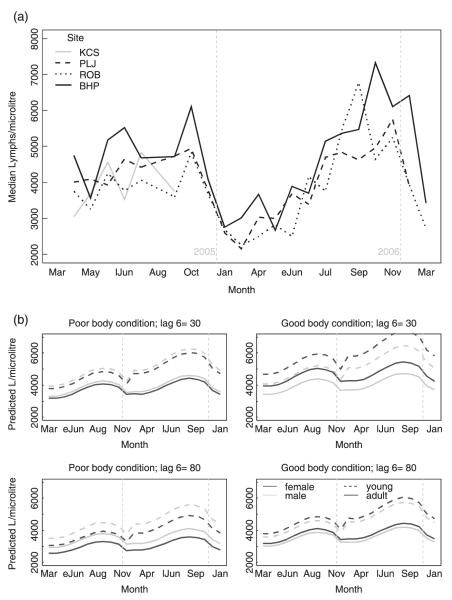
In wild field voles (Table 3), the seasonal pattern was consistent for all sites: low counts during winter and spring and higher counts in summer and autumn (Fig. 2). Lymphocyte counts tended to decline as voles aged. Past density (6 months previously) had a substantial negative effect on lymphocytes, especially in females. In them, low lymphocyte counts were also associated with poor body condition. For reproductive females, the effect of breeding varied with season. While the seasonal pattern in non-gravid females was as described above, the increase in counts as the breeding season progressed was only slight for lactating females, and counts in gestating females decreased. Thus, at the beginning of the breeding season, gestating females had the highest counts, but by the end of the season they were the lowest. Again, the contrast with the animal house results suggests that females with higher lymphocyte counts tended to start reproduction earlier.
Table 3.
Mixed-effects models describing factors associated with the variation of lymphocytes in all post-juvenile field voles year-round, and in adult females during the breeding season
| Term | Coefficients | SE | P-value | 95% CI for coefficients* |
ΔAIC† |
|---|---|---|---|---|---|
| Post-juveniles | |||||
| Model = L0·1~AGE + SEASON[sin] + SEASON[cos] + SEX + BODYCOND + YEAR + R/N + DENSITY-6 + SEX * BODYCOND + SEX * DENSITY-6 + AGE * YEAR + SEASON[cos] * R/N + AGE * R/N Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 2650 | |||||
| Intercept | 2·31 | 0·02 | < 0·0001 | 2·27, 2·35 | - |
| AGE_CLASS(adult) | −0·04 | < 0·0001 | −0·06, | −0·02 | - |
| SEASON[sin] | −0·007 | 0·008 | 0·3721 | −0·03, 0·01 | 0 |
| SEASON[cos] | −0·04 | 0·009 | < 0·0001 | −0·07, − 0·02 | - |
| SEX(m) | 0·036 | 0·013 | 0·1754 | −0·016, 0·08 | - |
| BODYCOND | 0·007 | 0·002 | 0·0021 | 0·003, 0·01 | - |
| YEAR(2nd) | 0·034 | 0·012 | 0·0053 | 0·0006, 0·06 | - |
| R/N(N) | 0·015 | 0·007 | 0·0331 | 0·002, 0·03 | - |
| DENSITY-6 | −0·0008 | 0·0002 | 0·0004 | −0·001, − 0·003 | - |
| SEX(m) * BODYCOND | −0·01 | 0·0045 | 0·0272 | −0·02, − 0·002 | 3·8 |
| SEX(m) * DENSITY-6 | 0·0004 | 0·00017 | 0·0207 | 0·0001, 0·0008 | 6·6 |
| AGE_CLASS(adult) * YEAR(2nd) | −0·03 | 0·01 | 0·0078 | −0·05, − 0·008 | 3·9 |
| SEASON[cos] * R/N(N) | −0·03 | 0·01 | 0·0014 | −0·05, − 0·01 | 6·5 |
| AGE_CLASS (adult) * R/N(N) | 0·04 | 0·01 | 0·0026 | 0·01, 0·06 | 7·2 |
| Adult females during breeding season | |||||
| Model = L0·1~REPR + SEASON[cos] + SEASON[sin] + DENSITY-6 + REPR * SEASON[cos] Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 515 | |||||
| Intercept | 2·30 | 0·02 | < 0·0001 | 2·27, 2·33 | - |
| REPR(gestating) | −0·039 | 0·016 | 0·0184 | −0·07, − 0·007 | - |
| REPR(lactating) | −0·058 | 0·019 | 0·0279 | −0·09, − 0·02 | - |
| SEASON[sin] | 0·029 | 0·016 | 0·0662 | −0·0006, 0·06 | - |
| SEASON[cos] | −0·018 | 0·015 | 0·2309 | −0·05, 0·006 | - |
| DENSITY-6 | −0·001 | 0·0002 | 0·0001 | −0·0015, − 0·0006 | 15 |
| REPR(gestating) * SEASON[cos] | 0·076 | 0·023 | 0·0009 | 0·03, 0·12 | 7 |
| REPR(lactating) * SEASON[cos] | 0·022 | 0·027 | 0·5270 | −0·03, 0·07 | 7 |
Fig. 2.
Lymphocyte counts in wild field voles. (a) Observed seasonal variation of field vole lymphocytes by trapping site. (b) Monthly predictions of lymphocyte levels for field voles of average body condition score for 32 scenarios: comparison between sexes (line colour), years (2005 and 2006), past densities (lag 6: 6 months previously), body condition scores (poor = 4; good = 8) and age (line type). (eJun = early June; lJun = late June).
Again, the two sampling years differed. Lymphocyte counts were higher for young voles, and the seasonal pattern more marked, in 2006.
NEUTROPHILS
In the captive colony, neutrophil counts were higher in males and increased as they aged, but there was no change with age in females (interaction P = 0·044).
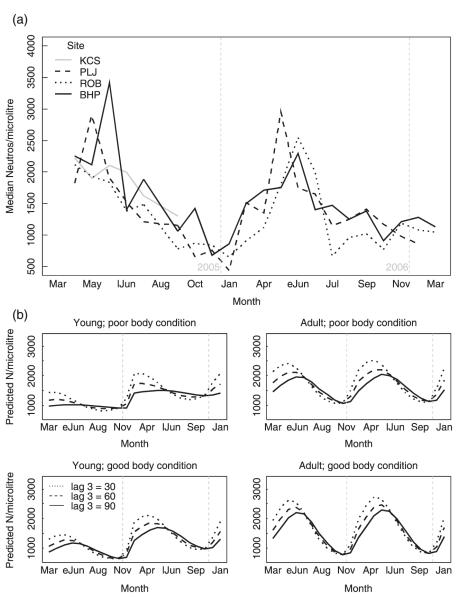
In the field, all trapping sites showed a clear seasonal pattern of high counts in spring and lower counts the rest of the year, reaching minimum values in the winter (Fig. 3; Table 4). Individuals in better body condition, especially adults, had higher peak counts in spring and the lowest counts in winter. When past densities (3 months previously) were high, peak neutrophil counts in spring were lower and increases were seen later. Males had higher counts than females. Lactating and gestating animals had consistently higher counts than non-gravid females. Finally, while young voles hardly showed a peak in spring 2005, the peak was much more pronounced in the second year.
Fig. 3.
Neutrophil counts in wild field voles. (a) Observed seasonal variation of field vole neutrophils by trapping site. (b) Predicted neutrophil levels for male field voles by month for 24 scenarios: comparison between years (2005 and 2006), past density (line type; lag 3: density 3 months previously), body condition score (poor = 4; good = 8) and age (eJun = early June; lJun = late June).
Table 4.
Mixed-effects models describing factors associated with the variation of neutrophils all post-juvenile field voles year-round, and in adult females during the breeding season
| Term | Coefficients | SE | P-value | 95% CI for coefficients* |
ΔAIC† |
|---|---|---|---|---|---|
| Post-juveniles | |||||
| Model = N0·1~AGE + SEASON[sin] + SEASON[cos] + SEX + BODYCOND + YEAR + DENSITY-3 + SEASON[sin] * BODYCOND + AGE * YEAR + SEASON[cos] * DENSITY-3 + AGE * SEASON[sin] Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 2650 | |||||
| Intercept | 2·09 | 0·02 | < 0·0001 | 2·03, 2·15 | - |
| AGE_CLASS(adult) | 0·047 | 0·008 | < 0·0001 | 0·03, 0·06 | - |
| SEASON[sin] | −0·008 | 0·025 | 0·7406 | −0·06, 0·05 | - |
| SEASON[cos] | 0·09 | 0·02 | < 0·0001 | 0·04, 0·14 | - |
| SEX(m) | 0·02 | 0·008 | 0·0085 | 0·005, 0·036 | 4·9 |
| BODYCOND | −0·005 | 0·003 | 0·0900 | −0·01, 0·001 | - |
| YEAR(2nd) | 0·08 | 0·02 | 0·0005 | 0·04, 0·11 | - |
| DENSITY-3 | −0·0004 | 0·00025 | 0·1136 | −0·001, 0·0003 | - |
| SEASON(sin) * BODYCOND | 0·01 | 0·004 | 0·0029 | 0·004, 0·02 | 8·9 |
| AGE_CLASS(adult) * YEAR(2nd) | −0·07 | 0·016 | 0·0002 | −0·095, − 0·038 | 15·3 |
| SEASON[cos] * DENSITY-3 | −0·001 | 0·0002 | 0·0001 | −0·0017, − 0·0003 | 14·7 |
| AGE_CLASS(adult) * SEASON[sin] | 0·05 | 0·01 | < 0·0001 | 0·031, 0·075 | 23·6 |
| Adult females during breeding season | |||||
| Model = N0·1~REPR + SEASON[cos] + SEASON[sin] Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 515 | |||||
| Intercept | 2·06 | 0·02 | < 0·0001 | 2·02, 2·11 | - |
| REPR(gestating) | 0·063 | 0·02 | 0·0011 | 0·03, 0·10 | |
| REPR(lactating) | 0·066 | 0·02 | 0·0010 | 0·03, 0·11 | 11 |
| SEASON[sin] | 0·03 | 0·03 | 0·283 | −0·02, 0·09 | 0 |
| SEASON[cos] | 0·08 | 0·02 | 0·0003 | 0·03, 0·12 | 11 |
MONOCYTES
In the experimental colony monocyte counts declined until approximately 100 days of age, and afterwards remained stable (age P = 0·030; age2 P = 0·033).
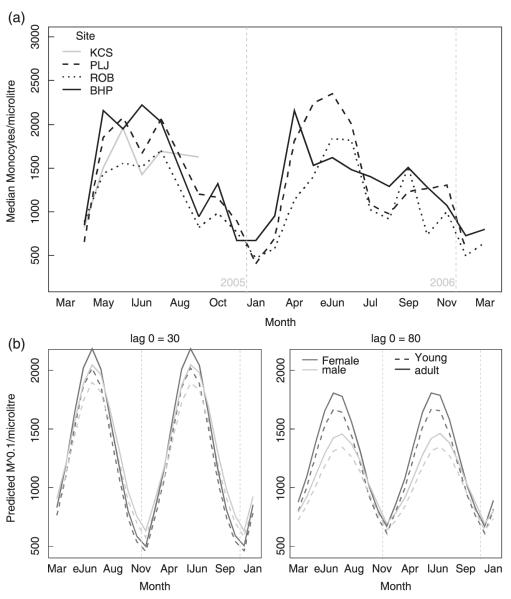
Monocyte counts showed a seasonal pattern consistent for all sites and very similar to that for neutrophils, except that they increased and decreased later in the year (Fig. 4; Table 5). Adults had higher counts than younger individuals, and counts were higher in individuals with better body condition. High contemporary densities (DENSITY-0) were associated with lower peak counts but also with higher troughs in winter. When vole density was also high, females had higher peak counts than males.
Fig. 4.
Monocyte counts in wild field voles. (a) Observed seasonal variation of field vole monocytes by trapping site. (b) Predicted monocyte levels for field voles of average body condition score, by month for 8 scenarios: comparison between current density (lag0), age (line type) and sex (line colour) (eJun = early June; lJun = late June).
Table 5.
Mixed-effects models describing factors associated with the variation of monocytes in all post-juvenile field voles year-round, and in adult females during the breeding season
| Term | Coefficients | SE | P-value | 95% CI for coefficients* |
ΔAIC† |
|---|---|---|---|---|---|
| Post-juveniles | |||||
| Model = M0·1~AGE + SEASON[sin] + SEASON[cos] + SEX + BODYCOND + R/N + DENSITY-0 + SEX * DENSITY-0 + SEASON[sin] * SEX + SEASON[sin] * DENSITY-1 Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 2650 | |||||
| Intercept | 1·97 | 0·03 | < 0·0001 | 1·9, 2·0 | - |
| AGE_CLASS(adult) | 0·016 | 0·007 | 0·0148 | 0·003, 0·03 | 4 |
| SEASON[sin] | 0·16 | 0·03 | < 0·0001 | 0·097, 0·24 | - |
| SEASON[cos] | −0·05 | 0·01 | 0·0002 | −0·08, − 0·02 | 12·2 |
| SEX(m) | 0·036 | 0·02 | 0·0856 | −0·006, 0·07 | - |
| BODYCOND | 0·009 | 0·002 | 0·0004 | 0·004, 0·01 | 11·7 |
| R/N(N) | 0·02 | 0·007 | 0·0015 | 0·009, 0·036 | 7·4 |
| DENSITY-0 | −0·0001 | 0·0004 | 0·6941 | −0·001, 0·0003 | - |
| SEX (m) * DENSITY-0 | −0·0007 | 0·0002 | 0·0051 | −0·0012, 0·0002 | 4·4 |
| SEASON[sin] * SEX(m) | −0·03 | 0·01 | 0·0032 | −0·052, − 0·01 | 4·2 |
| SEASON[sin] * DENSITY-1 | −0·001 | 0·0004 | 0·0035 | −0·002, − 0·0002 | 6·2 |
| Adult females during breeding season | |||||
| Model = M0·1~REPR + SEASON[cos] + SEASON[sin] + YEAR + DENSITY-0 + SEASON[cos] * YEAR + SEASON[sin] * DENSITY-0 + SEASON[cos] * DENSITY-0 Random effects = SITE * SESSION; VOLE_ID | |||||
| n = 515 | |||||
| Intercept | 1·86 | 0·06 | < 0·0001 | 1·72, 1·99 | - |
| REPR(gestating) | −0·0008 | 0·01 | 0·9593 | −0·03, 0·03 | 1·6 |
| REPR(lactating) | −0·03 | 0·016 | 0·0682 | −0·06, − 0·006 | 1·6 |
| SEASON[sin] | 0·34 | 0·07 | < 0·0001 | 0·19, 0·54 | - |
| SEASON[cos] | 0·03 | 0·04 | 0·4354 | −0·09, 0·10 | - |
| YEAR(2nd) | 0·037 | 0·02 | 0·0711 | −0·01, 0·08 | - |
| DENSITY-0 | 0·001 | 0·0007 | 0·0785 | −0·0003, 0·003 | - |
| SEASON[cos] * YEAR(2nd) | 0·12 | 0·03 | 0·0001 | 0·04, 0·19 | 12 |
| SEASON[sin] * DENSITY-0 | −0·003 | 0·0008 | 0·0003 | −0·005, − 0·001 | 9 |
| SEASON[cos] * DENSITY-0 | −0·002 | 0·0008 | 0·0186 | −0·004, 0·0005 | 3 |
SAMPLING YEARS
For the light that may be thrown on the causes of the haematological dissimilarities between the two sampling years, the main biologically relevant differences observed are described briefly here, with reference to the abundance cycle. Populations peaked in 2003 and crashed in 2004 at all sites (see Fig. S1 in online supplementary material), and densities remained low throughout that year. Population sizes were very low in March 2005 (< 16 per grid), immediately prior to sampling for the work reported here, but reproduction began by the end of that month and the populations built up quickly, reaching 100 or more individuals per grid in the summer. In March 2006 the number of individuals per grid ranged from 32 to 77, but soon after voles began breeding two of the sites crashed (PLJ and ROB). That spring, newborns grew 7·5 g per month on average, compared to 15·9 g in 2005 (unpublished data). By summer 2006 the difference in weight gain was much less marked (8·8 g and 5·1 g per month for 2005 and 2006, respectively). Colder ambient temperatures were registered during the first half of spring 2006 (average minimum temperatures in 2005 were 3·9 °C, 2·6 °C, 4·5 °C and 8·2 °C, for March, April, May and June, respectively, compared to −0·7 °C, 0·4 °C, 4·3 °C and 6·5 °C in 2006).
Discussion
The large quantities of data and number of variables examined here have generated a correspondingly large number of results, but for succinctness we focus upon the most ecologically significant conclusions. (1) All blood cell types showed a marked seasonal variation not explained by the other variables evaluated. (2) Three ‘physiological’ seasons are well delimited and characterized; (a) indicators of immunological investment appeared lowest in winter (lowest lymphocyte counts), but red blood cell counts were at their highest and indicators of inflammatory response at their lowest; (b) spring was characterized by a fall in red blood cell counts and peaks in indicators of inflammatory response; and (c) during the course of the third season, summer—autumn, red blood cell counts recovered, immunological investment measures increased and the indicators of inflammatory response decreased. (3) Poor body condition appeared to affect the inflammatory response (lower neutrophil and monocyte peaks) and the immunological investment (lower lymphocyte counts). (4) The first pregnant females of the year were those in better condition (high lymphocyte and red blood cell counts). (5) All the haematological parameters were affected by high population densities: counts were lower at times of high host densities (monocytes), or following high host densities (all other types). (6) Even when RBC counts were ‘high’ in the field, they were lower than in the near-optimal conditions of the animal house (abundant food and low parasitism/infection), suggesting that voles in the natural populations were generally resource and/or energy-limited, and they could therefore only increase their investment in, for instance, neutrophils by a compensatory decrease in their investment in other functions (e.g. the production of RBCs).
GENERIC INDICES OF HEALTH IN WILDLIFE
A variety of factors can cause fluctuations in blood cell counts (Table 1). Therefore, complementary information is required to interpret any pattern observed. With wildlife species, a lack of reference values may be overcome by generating data from a captive colony (as here), but problems of selection bias may remain. For example, neutrophil counts may have been highest in spring either because animals were especially prone to bacterial infection, or because it was only then that infected voles managed to survive such infections. Furthermore, it may be difficult to separate differential exposure from differential response. For example, the differences in neutrophil and monocyte counts between males and females might be attributable to different infection incidences, or to unequal capacities to mount an inflammatory response.
However, to pursue this particular example, the following observations suggest that the increase in neutrophil counts in early spring is, indeed, attributable to increased incidence of infection. First, the lower average counts in captive voles demonstrate that the increased counts in wild animals in spring are real, not simply a return to ‘normality’. Secondly, the cause of this neutrophilia in spring is unlikely to be epinephrine- or glucocorticoid-mediated stress, as the accompanying dynamics of lymphocytes are not compatible with those expected to be produced by stress of either kind (Feldman et al. 2000; Stockham & Scott 2002). Stress, furthermore, would give rise to a coincident (not delayed) increase in monocyte counts. Finally, the dynamics of RBC and lymphocyte counts in spring do not suggest that the neutrophilia reflects a generally improved immune response or a higher resistance to infections (selection bias) at this time.
However, offering such a detailed explanation to compare every hypothesis raised to alternative hypotheses is not practicable. Hence, we offer only the explanations that were considered most suitable, except where alternative hypotheses are similarly probable.
SEASONAL DYNAMICS OF HAEMATOLOGICAL PARAMETERS
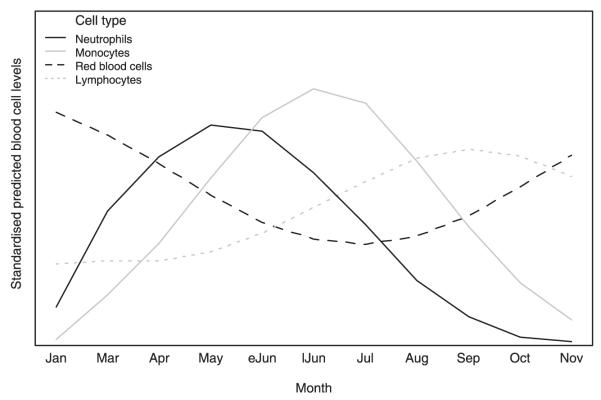
Figure 5 summarizes the seasonal trends for all cell types as predicted by the LMMs. Season appeared to be the main driver of haematological dynamics, with the highest levels of RBCs in winter and the lowest in spring, as also reported for other rodent species (e.g. Newson & Chitty 1962; Sealander 1964; Mira & Mathias 1994), and lowest lymphocyte counts in winter and high counts in summer and autumn, matching the pattern observed for lymphoid tissue in other small mammals (reviewed in Nelson & Demas 1996). The peak counts of monocytes and neutrophils during spring have not been reported by previous studies.
Fig. 5.
Seasonal trends for average levels of each cell type as predicted by the relevant linear mixed effects model (LMM). The vertical scale represents levels standardized to their respective minimum and maximum average levels, which allowed the fluctuations to be integrated into the same graph. The predictions were made for a recaptured adult male of average body condition, with host densities fixed at 50 individuals per grid.
In winter, field voles overcome food deficit by reducing their size (Ergon et al. 2004), which provides nutrients and reduces maintenance costs but hampers thermoregulation (Schmidt-Nielsen 1984), and so a compensatory increase in metabolism is required for overwinter survival (Jackson, Trayhurn & Speakman 2001). The resulting increased demand for oxygen has consistently been the explanation proposed for high RBC levels in rodents in winter (e.g. Sealander 1964; Mira & Mathias 1994), and such an argument is also pertinent here. However, winter RBC levels were still lower than those of non-breeding voles kept in captivity at 20 °C, suggesting that voles were nevertheless limited in their ability to respond to the stimulus of increased oxygen demands. This use of energy for thermogenesis is therefore also likely to decrease the availability of resources for other metabolically costly physiological processes, such as immune function (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000), as suggested by the low lymphocyte levels seen in winter. However, the incidence of infection appeared to be low (neutrophils and monocytes at their lowest levels), and the prevalence and burdens of parasites are also low at this time of the year (Bown et al. 2003; Smith et al. 2005). Hence, keeping the immunological investment to a minimum may not have affected survival greatly. Alternatively, low monocyte and lymphocyte levels might suggest insufficient immunocompetence to mount an inflammatory response during this period and/or death of those that become infected (selection bias), but this is unlikely as winter survival rate is generally high (Newson & Chitty 1962; Telfer et al. 2002).
In early spring, field voles show a marked increase in activity linked to the establishment of territories (Agrell et al. 1996) and they begin reproduction, both of which increase the rate of interactions with conspecifics and the environment. This, combined with the low immunological investment carried over from winter, may enable pathogens to circulate, causing in turn the steep rise in neutrophil counts that accompanies the onset of breeding, followed by a similar increase in monocytes, compatible with acute (neutrophils) and chronic (monocytes) inflammatory responses.
Besides the metabolic burden of breeding and mounting an inflammatory response (Bronson 1989; Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000), field voles increase their body size in early spring. Hence, this season generates an extremely high nutrient demand. The rapid decline in RBC levels that occurred from late March to July, in the face of competing metabolic demands but in spite of the high energy requirements, suggests that this period is likely to be an especially vulnerable one for the voles.
Others have also identified spring as critical to the dynamics of vole populations (e.g. Taitt & Krebs 1983). In a study on similar field vole populations, survival rates were lowest in spring (Newson & Chitty 1962), as were those of bank voles and wood mice in the United Kingdom (Telfer et al. 2002). Indeed, this ‘spring decline’ has been documented for a number of rodent species. The increased mortality has been attributed mainly to social factors and secondarily to dispersal (Agrell 1995), but the present study emphasizes that the interaction between disease and energetic deficit may also play an important role.
The spring drop in RBCs was especially marked during 2006. That year, too, young individuals had much higher neutrophil counts, suggesting that the new cohorts were facing more infections (and hence more energetic demands). Also, in 2005 heavier individuals had higher RBC counts, as described for laboratory rodents (Feldman et al. 2000), but the opposite was observed in 2006, which suggests that individuals with higher maintenance costs were suffering more. Combined with the difference in weight gain of newborns (above), all this suggests strongly that during spring 2006 either the quality and quantity of forage was lower than in 2005 or the metabolic demands were greater (thermogenesis, more infection, etc.), or both.
Abrupt changes were seen around July. Monocyte levels experienced a drastic drop, and counts of neutrophils had already started a rapid decrease in late spring. Lymphocyte counts, which had been increasing slowly during spring, accelerated to reach their peak in October. The build-up of vegetation during spring may, in part, account for a gradual increase in investment in immunocompetence, which in turn may have caused the decline in pathogenic infections that led to a decrease in neutrophils and monocytes. In addition, because those individuals born during decreasing photoperiods do not breed that year and grow little until the following spring (Goldman 2003; Ergon et al. 2004), the energetic requirements of summer are lower for an increasing proportion of the population. Therefore, with the additional metabolic demands of spring absent (much less inflammatory response and growth, and a lesser proportion of the population breeding), RBCs experienced a drastic recovery. By the end of autumn, however, lymphocyte levels declined drastically, as forage became poorer and temperatures colder.
THE EFFECT OF HOST DENSITY
Huitu et al. (2007) observed that after a peak of host abundance, the physiological condition of overwintered field voles was poorer than that which followed lower host densities. This is in agreement with our results, as levels of all cell types investigated were correlated negatively with host density.
High past density was correlated negatively with lymphocyte and RBC counts. Most plausibly, this is a delayed effect on condition resulting from the depletion of food resources (Huitu et al. 2007), which is also supported by the association of low body condition with low lymphocyte counts. Also, neutrophil peaks in spring were lower when past density was high, and monocyte increments were lower at high current densities. Given that individuals with poorer body condition also produced lower peaks of both neutrophils and monocytes, and that mounting an inflammatory response is expensive (Waterer & Wunderink 2003), these associations of monocytes and neutrophils with density are more likely to reflect different magnitudes of immune response than different incidences of infection. Together, this indicates that high host density may affect the immune response adversely, having the potential to influence the dynamics of a population by increasing its vulnerability to infectious disease.
THE EFFECT OF REPRODUCTION
In our captive colony, reproduction had an impact only on indicators of condition (RBCs and lymphocytes). The effects on RBCs were only seen in females, while lymphocyte levels were lower in both sexes only for those breeding for the first time, suggesting that the first mating episode is more stressful. In the wild, the beginning of the breeding season was accompanied by an acute rise in neutrophil levels, suggesting that infection rates increase at the time of the onset of breeding. It is known that high past densities may delay the beginning of reproduction (Ergon 2003); this probably caused the density-dependent delay in neutrophil peaks. Food deprivation causes lower oestradiol titres and inhibition of breeding in female voles (Pierce, Iwueke & Ferkin 2007). Delays in the onset of breeding following high densities may therefore be caused by impoverished condition, which is supported by our finding that females with higher RBCs and lymphocytes (two cells found to be correlated negatively with past density) gestated first in the breeding season. Regardless of the time of year, lactating and gestating females had higher neutrophil levels than non-gravid ones, perhaps reflecting an increased risk of infection, as in the animal house such a difference was absent.
Conclusions
The cause of periodic (multi-annual) peaks and declines in field vole populations remains unexplained (Mihok, Turner & Iverson 1985; Graham & Lambin 2002; Smith et al. 2006), but the present study provides an array of previously neglected avenues worth exploring. The strong seasonal variation in health dynamics pinpoints periods of increased vulnerability, both to infection and other causes of mortality. Some reviews have emphasized the rigours that rodents have to endure during winter (e.g. Ergon et al. 2004), but our results suggest that spring is when hardship is greatest. Low survival in spring has been considered mainly to be a consequence of changes in social behaviour (Taitt & Krebs 1983), but we provide reasons to expect low intrinsic survival during this period.
The immunological investment is low in winter, and low immunocompetence generally leads to increased severity of disease. For instance, cowpox virus infections have a negative effect on survival in bank voles and wood mice only in the winter (Telfer et al. 2002); but infection levels appear to be low at this time of the year. Hence, in winter, voles seem to be more vulnerable to infections but less at risk. Any circumstance that increased the risk of infection in winter months would have a large disease-induced impact on vole population dynamics.
Energetics seem to be vital (Ergon et al. 2004), particularly during early spring when sudden increases in metabolic requirements cannot be matched by forage growth. Studies in Kielder Forest have shown that past population densities influence the dates at which the reproductive season starts (Ergon 2003), and mathematical models have demonstrated that breeding season length alone has the potential to induce population cycles (Smith et al. 2006). Our results reinforce this by showing that past population densities affected indices of health and that only females in better condition began reproduction early. In addition, it was shown that the density-dependent effect that delays the onset of the breeding season also reduces resistance to infections and host fitness in general, potentially compromising population growth that year.
In summary, host condition in spring may not only reflect but may also, in part, determine whether a year will be in an increase or a decrease phase of the abundance cycle. Poor condition in over-wintering field voles is often a consequence of past densities (also suggested by Huitu et al. 2007), but it could also be caused by unusual increases of metabolic demands during spring, or poor resources bequeathed by a severe winter. This may help to explain why population cycles do not appear entirely regular.
Further studies that follow haematological levels of individual animals and contrast them with infection status with selected pathogens should be conducted to assess the role of immunity on infection dynamics. Investigation should also focus upon more specific immunological measures, and experimental approaches should be encouraged to confirm the causes of the patterns observed.
Supplementary Material
Fig. S1. Population size estimates for four field vole populations of Kielder Forest, from 2002 to 2006.
Table S1. Haematological values (expressed as median, and 5th and 95th percentiles) of free-ranging and captive field voles, grouped by sex, age and reproductive activity.
Acknowledgements
We thank Gill Telford, Roslyn Anderson, Jenny Rogers and Gemma Chaloner for their assistance in the field, and Sue Jopson for her work in the animal house. This work was funded by Wellcome Trust grant 075202/Z/04/Z to MB and a NERC Dorothy Hodgkin Award to P. B.
Footnotes
Supplementary material The following supplementary material is available for this article.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2656.2008.1413.x
(This link will take you to the article abstract.)
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agrell J. A shift in female social organization independent of relatedness: an experimental study on the field vole (Microtus agrestis) Behavioural Ecology. 1995;6:182–191. [Google Scholar]
- Agrell J, Erlinge S, Nelson J, Sandell M. Shifting spacing behaviour of male field voles (Microtus agrestis) over the reproductive season. Annales Zoologici Fennici. 1996;33:243–248. [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;AC-19:716–723. [Google Scholar]
- Bates D. lmer, P-values and all that. r-help archives. 2006. Available at: https://stat.ethz.ch/pipermail/r-help/2006-May/094765.html.
- Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden N. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerging Infectious Diseases. 2003;9:63–70. doi: 10.3201/eid0901.020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson F. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- Burthe S, Telfer S, Begon M, Bennett M, Smith A, Lambin X. Cowpox virus infection in natural field vole, Microtus agrestis, populations: significant negative impacts on survival. Journal of Animal Ecology. 2008;77:110–119. doi: 10.1111/j.1365-2656.2007.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burthe S, Telfer S, Lambin X, Bennett M, Carslake D, Smith A, Begon M. Cowpox virus infection in natural field vole Microtus agrestis populations: delayed density dependence and individual risk. Journal of Animal Ecology. 2006;75:1416–1425. doi: 10.1111/j.1365-2656.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- Diggle P. Time Series: a Biostatistical Introduction. Clarendon Press; Oxford: 1990. [Google Scholar]
- Ergon T. Fluctuating life-history traits in overwintering field voles (Microtus agrestis) University of Oslo; 2003. PhD thesis. [Google Scholar]
- Ergon T, Speakman JR, Scantlebury M, Cavanagh R, Lambin X. Optimal body size and energy expenditure during winter: why are voles smaller in declining populations? American Naturalist. 2004a;163:442–457. doi: 10.1086/381940. [DOI] [PubMed] [Google Scholar]
- Feldman BF, Zinkl JG, Jain NC. Schalm’s Veterinary Hematology. Lippincot, Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- Goldman BD. Pattern of melatonin secretion mediates transfer of photoperiod information from mother to fetus in mammals. Science STKE. 2003;2003:E29. doi: 10.1126/stke.2003.192.pe29. [DOI] [PubMed] [Google Scholar]
- Graham IM, Lambin X. The impact of weasel predation on cyclic field vole survival: the specialist predator hypothesis contradicted. Journal of Animal Ecology. 2002;71:946–956. [Google Scholar]
- Huggins R. On the statistical analysis of capture—recapture experiments. Biometrika. 1989;76:133–140. [Google Scholar]
- Huitu O, Jokinen I, Korpimäki E, Koskela E, Mappes T. Phase dependence in winter physiological condition of cyclic voles. Oikos. 2007;116:565–577. [Google Scholar]
- Jackson D, Trayhurn P, Speakman J. Associations between energetics and over-winter survival in the short-tailed field vole Microtus agrestis. Journal of Animal Ecology. 2001;70:633–640. [Google Scholar]
- Kendall W, Nichols J. Estimating temporary emigration using capture—recapture data with Pollock’s robust design. Ecology. 1997;78:563–578. [Google Scholar]
- Kleinbaum D, Kupper K, Muller E, Nizam A. Applied Regression Analysis and Other Multivariable Methods. Duxbury Press; Boston: 1998. [Google Scholar]
- Lochmiller RL. Immunocompetence and animal population regulation. Oikos. 1996;76:594–602. [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Mihok S, Schwartz B. Anemia at the onset of winter in the meadow vole (Microtus pennsylvanicus) Comparative Biochemistry and Physiology A. 1989;94:289–304. doi: 10.1016/0300-9629(89)90551-3. [DOI] [PubMed] [Google Scholar]
- Mihok S, Turner BN, Iverson SL. The characterization of vole population dynamics. Ecology Monographs. 1985;55:399–420. [Google Scholar]
- Mira A, Mathias M. Seasonal effects on the hematology and blood plasma proteins of two species of mice Mus musculus domesticus and M. spretus (Rodentia: Muridae) from Portugal. Hystrix. 1994;5:63–72. [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Quarterly Review of Biology. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Newson J, Chitty D. Haemoglobin levels, growth and survival in two Microtus populations. Ecology. 1962;43:733–738. [Google Scholar]
- Pierce A, Iwueke I, Ferkin M. Food deprivation and the role of estradiol in mediating sexual behaviors in meadow voles. Physiology and Behavior. 2007;90:353–361. doi: 10.1016/j.physbeh.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger S. Unified maximum likelihood estimates for closed capture—recapture models using mixtures. Biometrics. 2000;56:434–442. doi: 10.1111/j.0006-341x.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- Richards SA. Dealing with overdispersed count data in applied ecology. Journal of Applied Ecology. 2008;45:218–227. [Google Scholar]
- Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important? Cambridge University Press; New York: 1984. [Google Scholar]
- Sealander JA. The influence of body size, season, sex, age and other factors upon some blood parameters in small mammals. Journal of Mammalogy. 1964;45:598–616. [Google Scholar]
- Sheldon B, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Smith A, Telfer S, Burthe S, Bennett M, Begon M. Trypanosomes, fleas and field voles: ecological dynamics of a host—vector—parasite interaction. Parasitology. 2005;131:355–365. doi: 10.1017/s0031182005007766. [DOI] [PubMed] [Google Scholar]
- Smith MJ, White A, Lambin X, Sherratt JA, Begon M. Delayed density-dependent season length alone can lead to rodent population cycles. American Naturalist. 2006;167:695–704. doi: 10.1086/503119. [DOI] [PubMed] [Google Scholar]
- Stockham S, Scott M. Fundamentals of Veterinary Clinical Pathology. Iowa State Press; Ames, Iowa: 2002. [Google Scholar]
- Taitt M, Krebs C. Predation, cover, and food manipulations during a spring decline of Microtus townsendii. Journal of Animal Ecology. 1983;52:837–848. [Google Scholar]
- Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M. Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos. 2005;109:317–322. [Google Scholar]
- Telfer S, Bennett M, Bown KJ, Cavanagh R, Crespin L, Hazel SM, Jones T, Begon M. The effects of cowpox virus on survival in natural rodent populations: increases and decreases. Journal of Animal Ecology. 2002;71:558–568. [Google Scholar]
- Tizard IR. Veterinary Immunology. An Introduction. Saunders; Philadelphia: 2004. [Google Scholar]
- Tompkins DM, Begon M. Parasites can regulate wildlife populations. Parasitology Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- Waterer G, Wunderink R. Science review: genetic variability in the systemic inflammatory response. Critical Care. 2003;7:308–314. doi: 10.1186/cc2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Population size estimates for four field vole populations of Kielder Forest, from 2002 to 2006.
Table S1. Haematological values (expressed as median, and 5th and 95th percentiles) of free-ranging and captive field voles, grouped by sex, age and reproductive activity.