Abstract
The GABAB receptor is a heterodimer of GABAB(1) and GABAB(2) subunits. There are two isoforms of the GABAB(1) subunit: GABAB(1a) and GABAB(1b). Recent studies with mutant mice suggest a differential role for the two GABAB(1) isoforms in behavioural processes. As pharmacological and genetic studies have implicated GABAB receptors in cognition we investigated the behaviour of GABAB(1a) −/− and GABAB(1b) −/− mice in different types of cognitive paradigms. GABAB(1a) −/− and GABAB(1b) −/− mice were both impaired relative to wildtype controls in a continuous spontaneous alternation behaviour test of working spatial memory. In contrast to the reported phenotype of GABAB(1) −/− mice, however, neither GABAB(1a) −/− nor GABAB(1b) −/− mice were deficient in a passive avoidance task. On the other hand, GABAB(1a) −/− mice were impaired in familiar and novel object recognition. We conclude that GABAB(1) isoforms contribute differentially to GABAB receptor-mediated cognitive processes.
Keywords: GABAB1 isoforms, spatial memory, passive avoidance, object recognition
GABAB receptors are metabotropic GABA receptors formed by the heterodimerization of GABAB(1) and GABAB(2) subunits. They modulate excitability as presynaptic hetero- and autoreceptors that inhibit the release of neurotransmitters. They are also expressed postsynaptically, where they induce inhibitory postsynaptic potentials via activation of inwardly-rectifying potassium channels (reviewed in [2]).
Many studies with GABAB receptor ligands have implicated the GABAB receptor in cognitive processes (reviewed in [4]). GABAB receptor antagonists improved performance in a number of different cognitive tests, for example hippocampal-dependent spatial learning and memory [11,20,26], and passive [19] and active avoidance [10]. In contrast, GABAB receptor agonists generally (but not always [6,9]) impair learning and memory in these tasks. In addition, deletion of the GABAB(1) receptor subunit profoundly impaired passive avoidance performance in a gene dose-dependent manner [24].
The GABAB(1) subunit is predominantly expressed in the CNS as one of two isoforms: GABAB(1a) or GABAB(1b) [2]. Currently no isoform-specific ligands exist with which to dissect their influences on cognitive processes. Recently, however, mutant mice deficient in either the GABAB(1a) or GABAB(1b) isoforms have been generated [29]. We have previously reported that mice deficient in the GABAB(1a) isoform were impaired in hippocampal LTP and in an object recognition task [29]. GABAB(1a)−/− mice also did not discriminate between paired and unpaired tones in a conditioned fear paradigm [25], and failed to acquire a conditioned taste aversion (CTA) to a novel saccharin solution paired with a LiCl-induced malaise [15]. GABAB(1b)−/− mice, in contrast, acquired CTA normally, but failed to extinguish their aversion up to 30 days later [15]. These studies indicate that the GABAB(1) isoforms convey specific and differential components of cognitive processes. However, the impact of the GABAB(1) isoforms on other types of cognitive processes has yet to be elaborated on.
In this study, we utilized GABAB(1a)−/− and GABAB(1b)−/− mice to examine the influence of the isoforms in three different types of cognitive paradigms. We first aimed to assess novel and familiar object recognition, thus repeating our previous studies with a larger cohort of mice [29]. We also examined the mice in a test indicative of spatial working memory, namely continuous spontaneous alternation behaviour (SAB). Finally passive avoidance was investigated for comparison with the reported impairment of GABAB(1) −/−mice, lacking both GABAB(1) isoforms [24].
The generation of GABAB(1a)−/−, GABAB(1b)−/− and wildtype mice has been described previously [15,29]. Briefly, a point mutation knock-in strategy was adopted whereby initiation codons for the respective isoforms were replaced with a stop codon. Mice were generated and maintained on a pure BALB/c genetic background. GABAB(1a)−/− and GABAB(1b)−/− mice used in the present experiments were derived from homozygous breeding of siblings originating from the founding heterozygotic mice, while homozygous wild-type controls for the GABAB(1) isoform mutant mice were derived from mating together wild-type siblings generated from GABAB(1a)+/− and GABAB(1b)+/− heterozygous breeding in accordance with the recommendations proposed by The Jackson Laboratory to obviate genetic drift and the formation of substrains (http://jaxmice.jax.org/geneticquality/guidelines.html). In order to reduce the influence of intermale aggression or social dominance hierarchies on cognitive performance, adult mice were singly housed with sawdust bedding, tissue paper nesting materials and one Mouse House® (Nalgene, Nalge Nunc International, Rochester, NY) per cage. Housing was at a temperature of 22–24°C in a 12 h light:dark cycle with lights on at 6.30 am. Food pellets and tap water were available ad libitum (except during experimentation). All experiments were conducted during the light cycle, using male mice only. All animal experiments were conducted according to the Swiss recommendations and guidelines and approved by the Basel-Stadt Cantonal Veterinary Authority.
Experimentally naïve, male wild-type (n =19), GABAB(1a)−/− (n = 15) and GABAB(1b))−/− (n = 18) mice (mean age 24 (± 0.4) weeks) were tested in an object recognition task as previously described [29]. The test was based on the original principles of Ennaceur and Delacour, which relies upon the natural tendency of rodents to attend to a novel object more than to a familiar one [8]. Briefly, mice were habituated overnight to a new cage (22 × 37 × 15 (h) cm, containing sawdust bedding and ad libitum standard food and water). The following day at time = 0 min a clean PVC disc (2 (h) × 5 (d) cm) was placed for 3 min in the centre of the cage. The disc was then removed and 10 min later replaced by a new, identical clean disc. This was repeated at time = 24 h. At time = 24 h + 10 min, a clean grey PVC cone (6 (h) × 5 (d) cm) was placed in the cage. All objects were presented for a duration of 3 min and were handled only with tissue paper to prevent contamination with odours. All sessions were recorded on video. The frequency of stretch attend postures (SAP, defined as head and shoulders distinctly extended towards the object, from any position within the cage) was scored at least twice for each mouse by a trained observer blinded to the animals’ genotypes. This parameter was chosen as it has been validated in-house as the most sensitive measure for the detection of cognitive enhancers (data not shown), and eliminates the bias of traditional measures introduced by play behaviour related to novel objects, for which BALB/c mice are noted [17]. The mean of duplicate scores for each animal were used for analysis. Data from one GABAB(1a)−/− mouse was eliminated due to stereotypic circling behaviour during the test which was unrelated to its attention towards the objects. No other mice in this test, or in other tests, displayed circling behaviour. Data were analysed within genotype using a 1-way repeated measures analysis of variance (ANOVA), followed by Fisher LSD post hoc comparisons as appropriate.
In a separate cohort of mice, continuous SAB was assessed in a Y-maze constructed from 3 walled arms (8 cm-long, 6 cm-wide) in a radial arrangement each ending in a 10 cm square [23]. A clean sheet of paper was placed under the maze and the walls wiped clean for each mouse, to reduce odor traces between animals. Wildtype (n = 12), GABAB(1a) −/− (n = 9) and GABAB(1b)−/− (n = 9) male mice (mean age 21 (± 0.6) weeks) were placed individually into the Y-maze facing the end wall of a randomly allocated arm, and allowed to explore for 5 minutes. The order of the arms (1, 2 or 3) visited were recorded. Overlapping triplets of 3 arm visits that included each of the three arms was counted as 1 complete spontaneous alternation. The number of alternations performed was divided by the total number of arms visited minus 2 (i.e. the number of total possible alternations) to obtain % spontaneous alternation (%SA). Data were analyzed as using 1-way ANOVA for the effect of genotype followed by Fishers LSD post hoc comparisons.
A one-trial step-through passive avoidance paradigm was performed as previously described [24]. Briefly, wildtype (n = 23), GABAB(1a) −/− (n = 17) and GABAB(1b) −/− (n = 18) mice (aged 23 (± 0.6) weeks) were placed in the light side of a two-compartment trough-shaped apparatus. The barrier between the compartments was opened, the time taken to break a photobeam located 10.5 cm inside the dark compartment was recorded automatically (training latency) and a 0.5 mA foot shock (Campden Instruments 521 C Source Shock, rectangular current wave) was immediately delivered though the floor and walls. The shock duration was a maximum of 5 s or until the animal escaped back into the light side. Animals with a training latency >150 s were not shocked and were excluded from further testing (one wildtype and two GABAB(1a) −/− mice). The retention test was performed 24 hours later and was identical to the training trial except no shock was delivered. Maximum latency allowed in the retention test was 300 s. Data were analysed using a 2-way repeated measures ANOVA, where the factors were genotype and time.
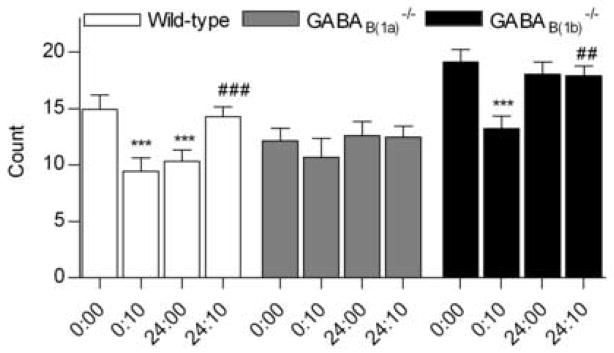
In the object recognition test, the number of SAPs of wildtype mice were affected by the different object presentation conditions (F3,72 = 9.45, P < 0.001; Fig. 1). The number of SAPs to the disc was decreased after a 10-min interval (P < 0.001) indicating that the mice remembered the object. Interestingly, the number of SAPs to the disc was also reduced 24 h later (P < 0.001). In our previous study, wildtype mice did not demonstrate recognition of the familiar disc after a 24-h interval [29]. Using a greater number of animals in the present study (n = 19 in this study versus 7 previously), it was clear that a more persistent memory trace of the object occurred in the wildtype mice. When presented with the novel cone at 24 hr 10 min, the number of SAPs increased relative to the disc at maximum recognition (i.e. to the 10 min disc presentation, P < 0.001) and to the disc presented at time = 24 h (P < 0.01), indicating that the mice recognised the cone as a novel object.
Fig. 1.
Number of stretch attend posture performed in an object recognition task by wildtype (n = 19), GABAB(1a) −/− (n = 14) and GABAB(1b) −/− (n = 18) mice towards a disc at initial presentation (Time = 0:00), to the same (clean) disc 10 min and 24 hr later (Time = 0:10 and 24:00, respectively) and to a novel cone-shaped object (Time 24:10). ***P < 0.001 vs Time 0:00 within genotype; ###P < 0.001, ##P < 0.01 versus Time 0:10 within genotype.
In contrast, no significant alterations were seen in the number of SAPs performed by GABAB(1a) −/− mice, irrespective of the delay between presentation of familiar objects, or of the novelty of the object (F3,55 = 0.70, P = 0.56; Fig. 1). This indicated that the GABAB(1a) −/− mice failed to recognise a familiar object, or to discriminate between the novel and familiar objects. This finding replicates that of our previous study [29].
The number of SAPs made by GABAB(1b) −/− mice towards the objects was influenced the different object presentation conditions (F3,71 = 7.35, P < 0.001; Fig. 1). Like the wild type mice, and in accordance with our previous findings, GABAB(1b) −/− mice recognised the familiar disc after a 10-min delay, as indicated by a reduction in the number of object-oriented SAPs (P < 0.001). However, similar to our original findings [29], the present study indicated that the GABAB(1b) −/− mice did not, on average, maintain a persistent memory trace of the disc (P = 0.43). In accordance with our previous findings, however, the ability of the GABAB(1b) −/− mice to discriminate between a familiar object (disc at 10 min) and a novel one (cone at 24 h 10 min) was intact (P = 0.001).
In the SAB test, both GABAB(1) isoform mutant strains were impaired relative to wildtype controls in the proportion of completed alternations (% SAB; F2,27 = 5.067, P < 0.05; Fig. 2). The number of arms entered, a measure of locomotor activity, was higher in the GABAB(1b) −/− mice than either the wildtype or GABAB(1a) −/− mice (P < 0.001 and P < 0.05, respectively; Fig. 2). Increased locomotor responses to a novel environment have been previously reported for GABAB(1b) −/− mice [14]. However, the greater number of arms visited by the GABAB(1b) −/− mice in this study did not improve the proportion of correct alternations in the SAB test. Although there are criticism of continuous SAB as a cognitive task per se [12], results of the present study suggest that both the GABAB(1) isoforms may have a role in working spatial memory. Further delineation of the roles of the isoforms in other more comprehensive tests of spatial working and reference memory are therefore warranted.
Fig. 2.
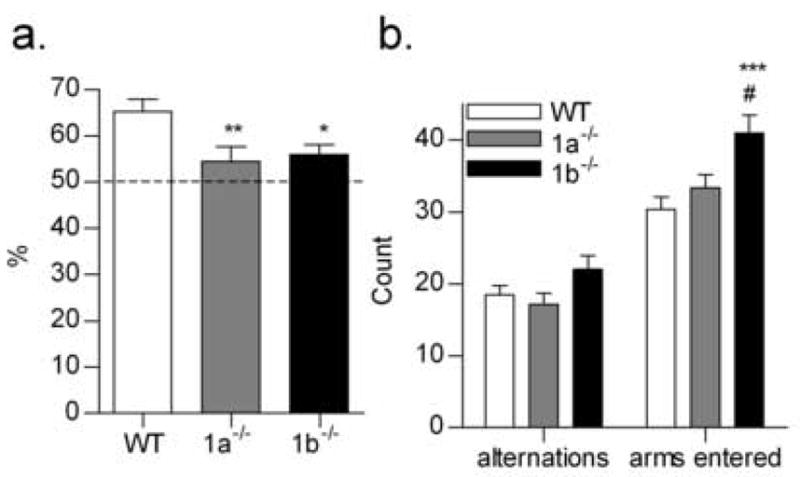
Percentage of correct alternations (a.), number of correct alternations and total number of arms visited (b.) by wildtype (WT, n = 12), GABAB(1a) −/− (1a−/−, n = 9) and GABAB(1b) −/− (1b−/−, n = 9) mice in a Y-maze continuous spontaneous alternation task. ***P < 0.001, **P < 0.01, * P < 0.05 versus WT; #P < 0.05 versus 1a−/−.
In passive avoidance training all mice vocalized on receiving the shock, indicating that pain sensory modalities were functional in both isoform-deficient mutant strains. Latency to enter the dark side was greater at retention testing than during training, although there was no effect of genotype on either training or retention latency (genotype F2,107 = 0.55, P = 0.58; training F1,107 = 77.11, P < 0.001; interaction F2,107 = 0.30, P = 0.74; Fig. 3). This indicated that the memory of the shock received in the dark compartment was functional in both mutant strains. This finding contrasts with that of the aforementioned GABAB(1) −/− mice, which lack both functional isoforms and show profound memory deficits in the retention phase of this test. [24]. It should be noted however, that in GABAB(1) −/− mice, GABAB receptor agonist-induced responses are completely abolished [16,24]. In contrast, in the GABAB(1a) −/− and GABAB(1b) −/− mice the loss of GABAB receptor function is partial [29], and some GABAB receptor agonist-induced functions remain in both of these mutant strains [14,21,25,29]. Together these findings indicate that heterodimeric GABAB receptor function is essential for the retention of passive shock-avoidance training, and that this may be accomplished with either one of the GABAB(1) isoforms.
Fig. 3.
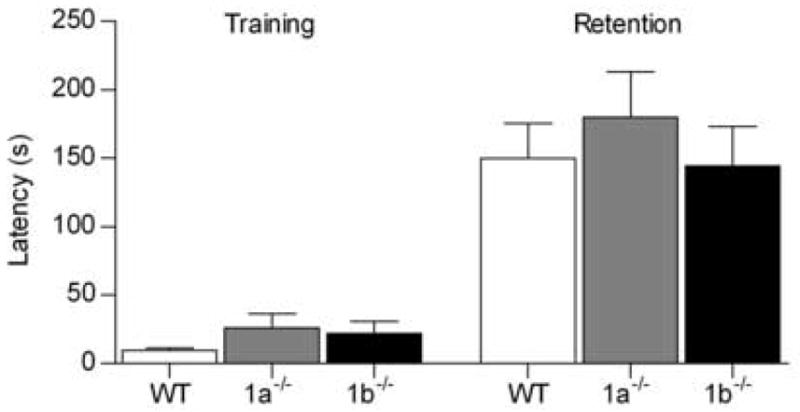
Latency of wildtype (WT, n = 23), GABAB(1a) −/− (1a−/−, n = 17) and GABAB(1b) −/− (1b−/−, n = 18) mice to enter the dark compartment during training and 24 h later during retention testing in a one-trial step-through passive avoidance paradigm.
The present study indicated that the GABAB(1) isoforms contribute differentially to distinct cognitive capabilities. Currently it is difficult to speculate about the neuronal and molecular mechanisms underlying these differences. The hippocampus is implicated in spontaneous alternation [18], object recognition [5,7] and passive avoidance [13,28]. As the GABAB(1a) −/− mice are deficient in hippocampal LTP [29], an impairment in this mutant in all three of these tasks could have been expected, although this was not the case as passive avoidance capabilities were preserved in these mice. However, it is also clear that learning can occur in mice in the absence of LTP (reviewed in [1]). Other brain regions are of course also important in all these tasks, for example the rhinal cortices are crucial for both passive avoidance (for example, [22]) and object recognition learning (reviewed in [27]). GABAB(1) isoforms, however, are widely expressed throughout the brain, and the isoforms diverge in expression profile both between and within various structures, including within the anatomical divisions of the hippocampus and neocortex [3]. Furthermore, the ultrastructural expression of the GABAB(1) isoforms differs markedly. In the hippocampus and lateral amygdala the GABAB(1a) isoform is a presynaptic heteroreceptor at glutamatergic terminals, while GABAB(1b) is mostly postsynaptically located, and both are autoreceptors [25,29]. This pattern is preserved to a certain degree in layer 5 pyramidal cortical neurons, with the exception that the autoreceptor is predominantly the GABAB(1a) isoform [21].
It is interesting to note that specific isoform deletion produced cognitive impairments in some tasks, which appears contrary to the cognitive impairing effects of GABAB receptor agonists. However, GABAB receptor agonists baclofen and GHB do not distinguish between GABAB(1) receptor isoforms [2,14], and hence fail to discriminate between GABAB receptors expressed presynaptically, postsynaptic or as autoreceptors. Therefore activation of GABAB receptors at all three sites concurrently can be expected to produce different responses in comparison to targeted isoform-specific genetic deletions.
To summarise, the present study demonstrated that the GABAB(1a) isoform is essential for object recognition and discrimination, while the GABAB(1b) isoform may be required more for either the retrieval or long-term storage of this type of memory. Impairments of both isoform-deficient mice in continuous SAB indicate that further examination of spatial working memory are warranted, and may confirm that both isoforms contribute to this cognitive capability. In contrast, although previous research has demonstrated that complete GABAB receptor heterodimers are required for effective passive avoidance performance, the present study shows that the presence of either of the GABAB(1) isoforms is sufficient for normal performance. Previous studies have indicated that cognitive performance may be influenced by epigenetic factors (for example [30]). Although we cannot draw conclusions about the potential influence of such factors in the present study, our results indicate that future research may benefit from pursuit of development of isoform-specific ligands ultimately for the development of cognitive therapeutics. We conclude that the GABAB(1a) and GABAB(1b) isoforms are molecular variants of the GABAB receptor system which contribute differentially to specific components of GABAB receptor-mediated cognitive functioning.
Acknowledgments
This work was supported by the National Institutes of Mental Health/National Institute on Drug Abuse grant U01 MH69062 (LHJ, KK, JFC) and the Swiss Science Foundation (3100-067100.01, BB). The authors thank Dr. Annick Vassout for discussions and Erich Müller, Christine Hunn and Charlotte Huber for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bannerman DM, Rawlins JN, Good MA. The drugs don’t work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology (Berl) 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- 2.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- 4.Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 5.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellano C, Cestari V, Cabib S, Puglisi-Allegra S. Strain-dependent effects of post-training GABA receptor agonists and antagonists on memory storage in mice. Psychopharmacology (Berl) 1993;111:134–138. doi: 10.1007/BF02245514. [DOI] [PubMed] [Google Scholar]
- 7.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 9.Escher T, Mittleman G. Effects of ethanol and GABAB drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- 10.Getova D, Bowery NG. The modulatory effects of high affinity GABA(B) receptor antagonists in an active avoidance learning paradigm in rats. Psychopharmacology (Berl) 1998;137:369–373. doi: 10.1007/s002130050632. [DOI] [PubMed] [Google Scholar]
- 11.Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, Gallagher M. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson LH, Bettler B, Kaupmann K, Cryan JF. GABAB(1) receptor subunit isoforms exert a differential influence on baseline but not GABAB receptor agonist - induced changes in mice. J Pharmacol Exp Ther. 2006;319:1317–1326. doi: 10.1124/jpet.106.111971. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26:8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, Brauner-Osborne H, Waldmeier P, Bettler B. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Chae S, Lee J, Yang H, Shin HS. Variations in the behaviors to novel objects among five inbred strains of mice. Genes Brain Behav. 2005;4:302–306. doi: 10.1111/j.1601-183X.2005.00133.x. [DOI] [PubMed] [Google Scholar]
- 18.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 19.Mondadori C, Jaekel J, Preiswerk G. CGP 36742: the first orally active GABAB blocker improves the cognitive performance of mice, rats, and rhesus monkeys. Behav Neural Biol. 1993;60:62–68. doi: 10.1016/0163-1047(93)90729-2. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Takashima T. The GABA(B) receptor antagonist CGP36742 attenuates the baclofen- and scopolamine-induced deficit in Morris water maze task in rats. Brain Res. 1997;766:101–106. doi: 10.1016/s0006-8993(97)00529-5. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABA(B1b) Isoform Mediates Long-Lasting Inhibition of Dendritic Ca(2+) Spikes in Layer 5 Somatosensory Pyramidal Neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alternation behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl) 1988;94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- 24.Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 25.Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- 26.Staubli U, Scafidi J, Chun D. GABAB receptor antagonism: facilitatory effects on memory parallel those on LTP induced by TBS but not HFS. J Neurosci. 1999;19:4609–4615. doi: 10.1523/JNEUROSCI.19-11-04609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steckler T, Drinkenburg WH, Sahgal A, Aggleton JP. Recognition memory in rats--II. Neuroanatomical substrates. Prog Neurobiol. 1998;54:313–332. doi: 10.1016/s0301-0082(97)00061-0. [DOI] [PubMed] [Google Scholar]
- 28.Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- 29.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential Compartmentalization and Distinct Functions of GABA(B) Receptor Variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaharia MD, Kulczycki J, Shanks N, Meaney MJ, Anisman H. The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: genetic and maternal factors. Psychopharmacology (Berl) 1996;128:227–239. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]