Fig. 1.
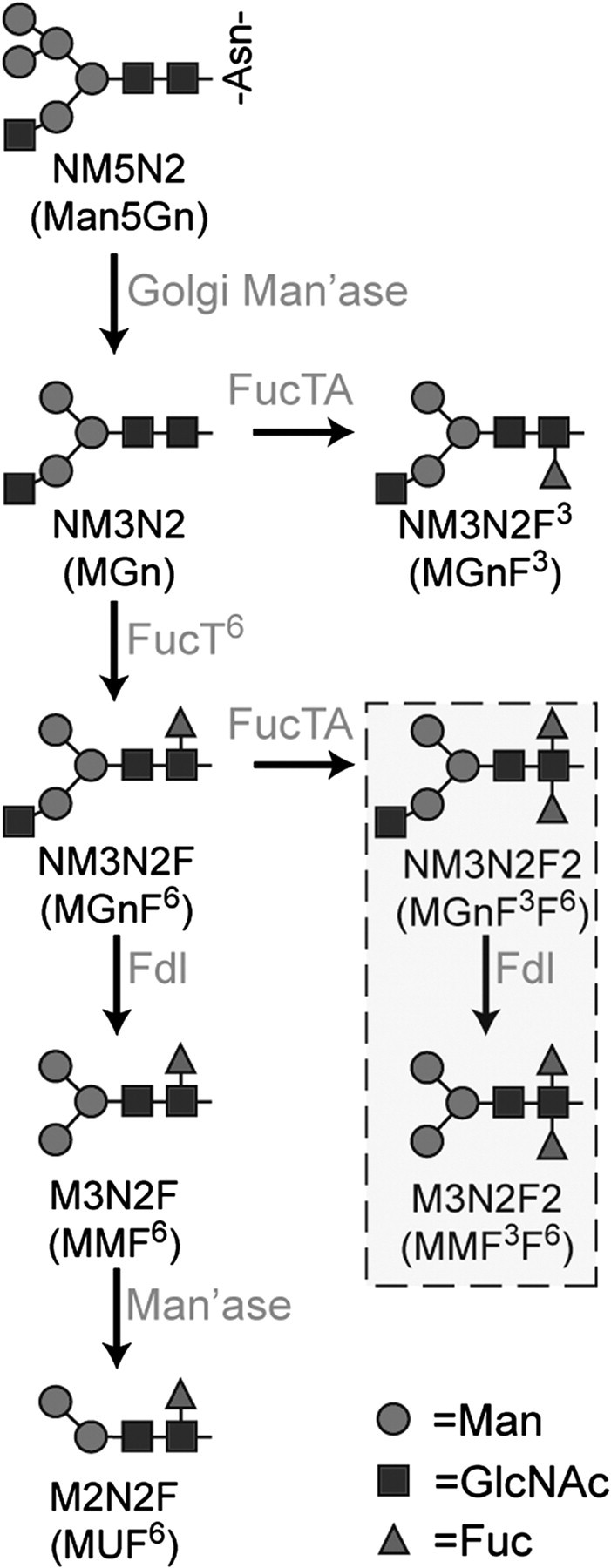
Pathways for N-linked glycan fucosylation in Drosophila. N-acetylglucosaminyltransferase I (GlcNAc-TI) adds N-acetylglucosamine to the high-mannose glycan Man5GlcNAc2 on glycoproteins transported to the Golgi, resulting in glycans with the composition Man5GlcNAc3. This octasaccharide is substrate for the production of all hybrid, complex, and paucimannose N-glycans in the Drosophila embryo. Two shorthand nomenclatures are in general use to describe glycan structures that derive from Man5GlcNAc3, also known as NM5N2 (Man5Gn; alternative names are in parentheses). Following trimming by Golgi mannosidases (Golgi Man’ase), the NM3N2 (MGn) glycan and its monofucosylated derivative NM3N2F6 (MGnF6) are substrates for production of the HRP epitopes, NM3N2F3 (MGnF3) and NM3N2F2 (MGnF3F6), which are found in very low prevalence in the embryo. The major pathway for HRP epitope synthesis is initiated by addition of fucose (Fuc) in α6-linkage to NM3N2 (MGn) by Fucosyltransferase 6 (FucT6), followed by addition of α3-linked Fuc, which is catalyzed by Fucosyltransferase A (FucTA) in vitro. FucTA prefers substrates already containing α6-linked Fuc; thus, most α3-fucosylated glycans are difucosylated. The hexosaminidase known as “Fused lobes” (Fdl) removes non-reducing terminal GlcNAc to produce paucimannose glycans such as M3N2F6 (MMF6) or M3N2F2 (MMF3F6). An unidentified mannosidase activity (Man’ase) removes an additional Man residue from the M3N2F6 (MMF6) structure to generate M2N2F6 (MUF6). The boxed glycans are a subset of the HRP epitopes that have been detected in Drosophila embryos, of which M3N2F2 (MMF3F6) is the most prevalent. Graphical representations of glycans are consistent with the nomenclature of the Consortium for Functional Glycomics.
