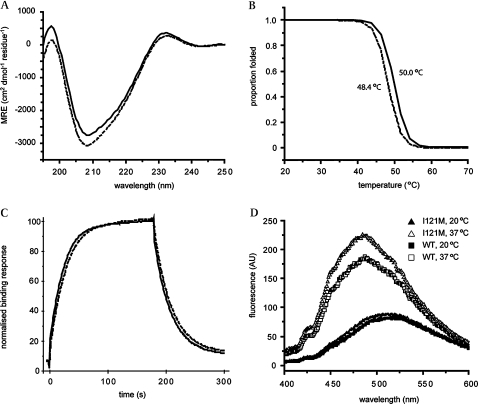
Figure 3.
Biophysical characterisation of the I121M variant T-box. (A) Far UV CD spectra of WT (solid line) and I121M variant (dashed line) TBX20 T-box domains indicate similar folded, β-sheet rich secondary structures. (B) Proportion of secondary structure folded as protein is heated. The I121M variant (dashed line) domain displays a ∼2°C reduction in Tm compared to WT (solid line), as measured by loss of CD signal at 215 nm. (C) Binding curves for I121M (dashed line) and WT (solid line) from representative surface plasmon resonance kinetic experiment. The mutation does not significantly affect the affinity of the domain for the T-site, as evidenced by very similar rates for binding and dissociation from the DNA displayed by both domains. (D) ANS fluorescence measured for I121M at 20°C and 37°C (solid and open triangles, respectively) and WT TBX20 T-box at 20 and 37°C (solid and open squares, respectively). Both domains show increased ANS binding at 37°C, a sign that tertiary contacts are relatively weak but the variant binds more ANS than the WT domain, indicating that the mutation destabilises the hydrophobic core further.