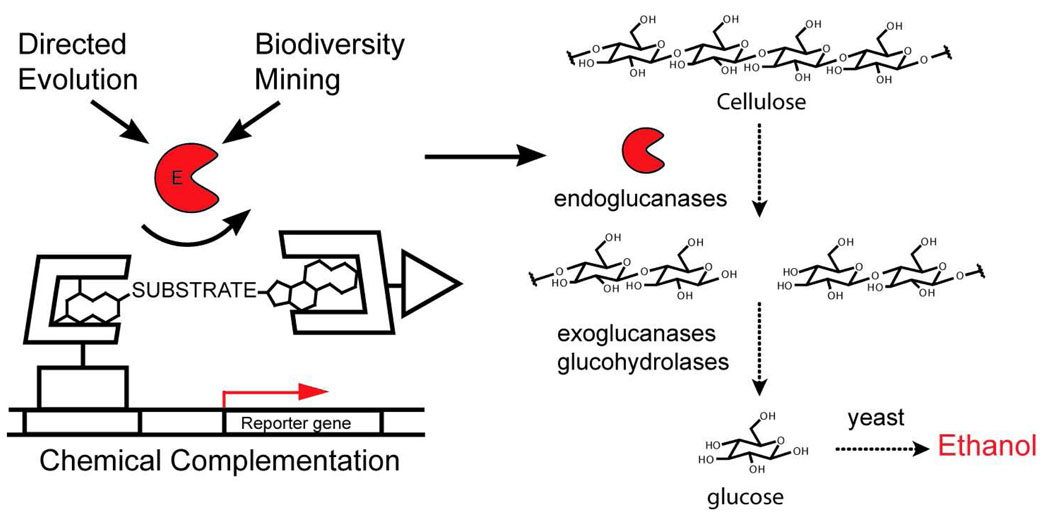
Figure 1.
Chemical complementation provides a high-throughput selection for cellulase catalysts. The discovery of improved cellulases, and other hydrolase enzymes important for biomass conversion is limited by existing medium-throughput screening technologies. Chemical complementation has been adapted to provide a high-throughput selection for bond cleavage reactions and specifically cellulose catalysts. Chemical complementation detects enzyme catalysis of bond formation or cleavage reactions based on covalent coupling of two small molecule ligands. The heterodimeric small molecule reconstitutes a transcriptional activator, turning on the transcription of a downstream reporter gene. Here, a dexamethasone (Dex)-methotrexate (Mtx) yeast three-hybrid system is used. Endoglucanase activity is detected as cleavage of a glycosidic linkage between Mtx and Dex. A cellulase selection could be used as an initial high-throughput assay to evaluate very large libraries of cellulase variants generated either by mutagenesis or isolated from biodiversity sources. Active cellulases emerging from the high-throughput assay could be then tested using low-throughput assays using plant material that more closely mimic industrial conditions.