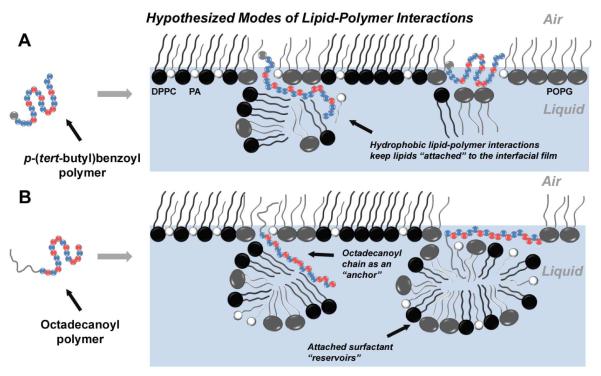
Figure 8. Hypothesized Modes of Lipid-Polymer Interactions.
The hypothesized lipid-polymer interaction(s) contributing to enhanced surface activity. For the polymers, blue spheres represent the lipophilic units while red spheres indicate cationic units. The gray sphere in the ‘polymer sequence’ of A represents the p-(tert-butyl)benzoyl end group, while the gray line extending from the ‘polymer sequence’ in B represents the octadecanoyl chain. Therefore, the picture outlines two possibilities of modes of action: one for polymers without an octadecanoyl chain (A), and one for octadecanoylated polymers (B). Panels A and B each represent a Tanaka lipid (TL, DPPC:POPG:PA) monolayer at the air-liquid interface, with black spheres representing DPPC, gray spheres representing POPG, and white spheres representing PA. Copolymers with a p-(tert-butyl)benzoyl end group (A) adopt an amphiphilic conformation and insert into the lipid film, where they are able to retain attached lipids via Coulombic interactions between cationic subunits and charged lipid head groups, or hydrophobic interactions between lipophilic subunits and the lipid acyl chains. However, octadecanoylated copolymers (B) could act as lipid “anchors” by increasing the degree of insertion into the lipid acyl chains through hydrophobic hydrocarbon chain-chain interactions. These polymers may also be able to sustain sublayer lipid structures by either adopting a lipid-associated amphiphilic conformation, or utilizing the octadecanoyl chain to retain pockets of lipid material.