Table 1.
Optimization of Conditions
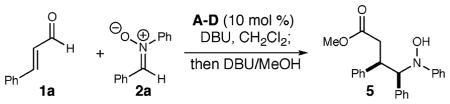 | |||||
|---|---|---|---|---|---|
| entry | azolium salt | temp (°C) | yielda (%) | drb | eec (%) |
| 1 | A | 0 | 75d | 4:1 | |
| 2 | B | 0 | 46d | 8:1 | −65 |
| 3 | C | 0 | 52d | 8:1 | −33 |
| 4 | D | 0 | 51d | 8:1 | 87 |
| 5 | D | −25 | 49d | 20:1 | 93 |
| 6 | De | −25 | 70f | 20:1 | 93 |
Isolated yields.
Diastereomeric ratio determined by 500 MHz NMR spectroscopy.
Enantiomeric excess determined by HPLC Chiracel AD-H.
2:1 ratio of 1a to 2a.
20 mol % of D, Et3N used instead of DBU.
2:1 ratio of 2a to 1a, NaOMe/MeOH used in place of DBU/MeOH.
