Table 2.
Nitrone Reaction Scope
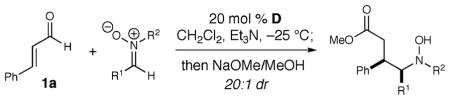 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | yielda (%) | eeb (%) |
| 1 | Ph | Ph | 70 (5) | 93 |
| 2 | 4-Me–C6H4 | Ph | 71 (6) | 90 |
| 3 | 4-Br–C6H4 | Ph | 68 (7) | 84 |
| 4 | 4-MeO-C6H4 | Ph | 62 (8) | 90 |
| 5 | 2-naphthyl | Ph | 69 (9) | 81 |
| 6 | cyclohexyl | Ph | 0 | |
| 7 | Ph | 4Cl-Ph | 80 (10) | 93 |
Isolated yields.
Enantiomeric excess determined by HPLC Chiracel OD-H or AD-H. Diastereomeric ratio determined by 1H NMR spectroscopy (500 MHz).
