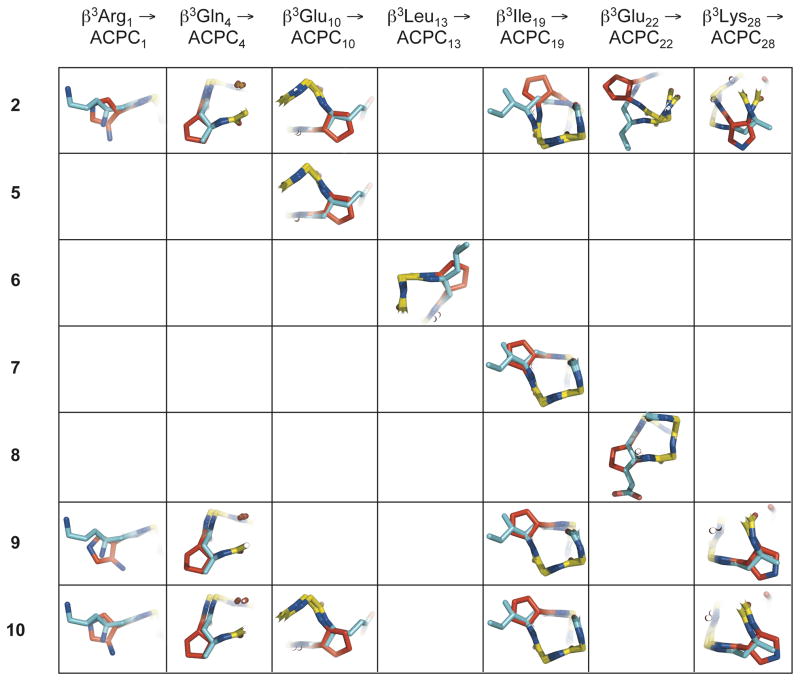
Figure 4.
Overlay of individual cyclic β-residues in α/β-peptides 2, and 5–10 with corresponding acyclic β3-residues in α/β-peptide 1, using the crystal structures of each peptide. These overlays were generated by aligning backbone atoms (amino N, Cβ, Cα, carbonyl C, carbonyl O, and for β-residues, Cβ) from residues 1–31 of α/β-peptides 2 and 5–10 with backbone atoms from the corresponding residues of α/β-peptide 1 (only one the two crystallographically distinct but nearly identical helix bundles from the structure of α/β-peptide 5 was included in the overlay analysis). Except for the ACPC and APC substitutions at β3Ile19, β3Glu22, and β3Lys28 in 2 (upper right), cyclic β-residues generally overlay well with their β3-residue counterparts, with backbone Cα and Cβ and side-chain Cγ atoms from each residue type located in close proximity. The close alignment of these structures suggests that cyclic β-residues are generally structurally conservative replacements for acyclic β3-residues in α/β-peptides. α-Amino acid residues are colored yellow, β3-amino acid residues are colored blue, and cyclic β-amino acid residues are colored red.