Table 1.
Reaction Optimization
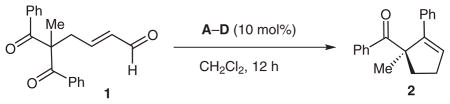 | |||||
|---|---|---|---|---|---|
| Entry | Temp (°C) | Basea | Azolium salt | Yieldb (%) | eec (%) |
| 1 | 23 | DBU | A | 0 | – |
| 2 | 23 | i-Pr2EtN | A | 47 | −83 |
| 3 | 23 | i-Pr2EtN | B | 38 | −76 |
| 4 | 23 | i-Pr2EtN | C | 45 | 51 |
| 5 | 23 | i-Pr2EtN | D | 40 | 94 |
| 6d | 23 | i-Pr2EtN | D | 66 | 94 |
| 7d | 40 | i-Pr2EtN | D | 80 | 93 |
| 8d,e | 40 | i-Pr2EtN | D | 70 | 93 |
1 equiv of base used.
Isolated yields.
Determined by HPLC (Chiralcel OD-H).
Careful exclusion of oxygen.
5 mol% of D.
