Table 2.
Substrate Scope
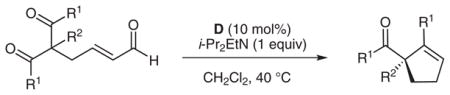 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | Cyclopentene | Yielda (%) | eeb (%) |
| 1 | Ph | Me | 2 | 80 | 93 |
| 2c | Ph | Me | 2 | 78 | 91 |
| 3 | 4-ClC6H4 | Me | 3 | 76 | 94 |
| 4 | 4-MeC6H4 | Me | 4 | 60 | 94 |
| 5 | 3-MeC6H4 | Me | 5 | 65 | 93 |
| 6 | 4-MeOC6H4 | Me | 6 | <5 | nd |
| 7 | Ph | Et | 7 | 73 | 90 |
| 8 | Ph | allyl | 8 | 70 | 83 |
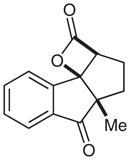
| 9 | 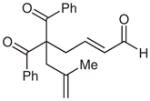 |
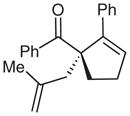 9 |
69 | 83 | |
| 10d | 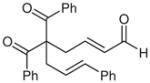 |
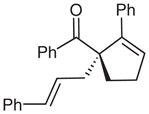 10 |
64 | 82 | |
| 11d | 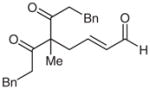 |
 11 |
65e | 93 | |
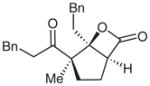
| 12d | 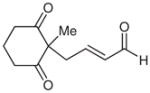 |
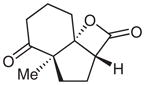 12 |
51e | 96 | |
| 13d | 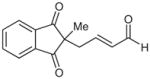 |
 13 |
36e | 15 | |
Isolated yields.
Determined by HPLC; nd = not determined.
Reaction run with 0.50 g of enal 1.
20 mol% of D.
Diastereomeric ratio 20:1. Relative and absolute stereochemistry determined by NOE and X-ray crystallography. See ref. 10 for details.
