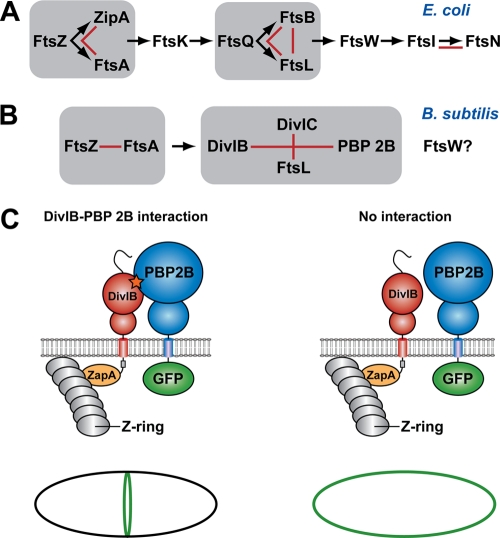
FIG. 1.
Schema showing the hierarchical pathway of divisome assembly in E. coli and B. subtilis (adapted from reference 30). For a protein to be recruited to the divisome, all of the proteins upstream from it in the hierarchical recruitment pathway must already be present at the septum. Groups of proteins that form a subcomplex independent of other divisomal proteins, such as the ternary complex formed between E. coli FtsQ, FtsB, and FtsL, are highlighted by gray boxes. Red lines denote pairwise protein-protein interactions that have been experimentally demonstrated using genetic and/or biochemical approaches. The question mark indicates that the precise location of FtsW in the divisome assembly pathway in B. subtilis is currently unknown. (C) Possible outcomes of a heterologous septal targeting experiment in E. coli in which ZapA-DivIB is employed as the bait and GFP-PBP 2B is the prey. A direct interaction between DivIB and PBP 2B should result in a fluorescent ring at midcell (or a pair of dots when viewed in cross-section) due the recruitment of GFP-PBP 2B to the divisome (left panel). In contrast, a halo of fluorescence should be visible around the cell periphery due to the membrane-bound GFP-PBP 2B if there is no interaction between these two proteins (right panel).