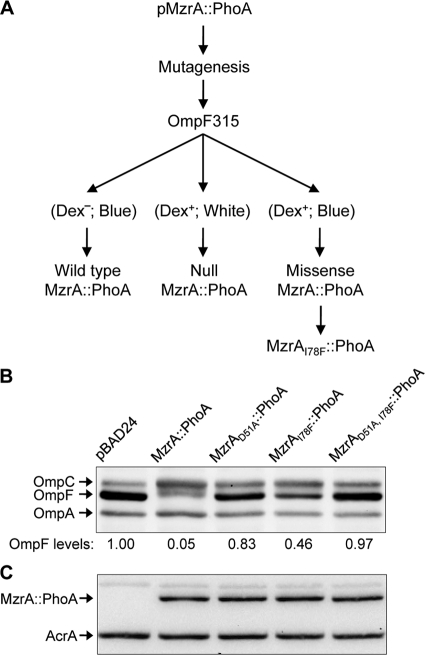
FIG. 6.
Isolation and characterization of a functionally inactive MzrA mutant. (A) Genetic strategy for isolating functionally null, nonpolar missense mutations in mzrA. The mzrA sequence, corresponding to amino acid residues 32 to 104 of the protein, from a plasmid expressing MzrA::PhoA fusion was subjected to random, megaprimer-based mutagenesis. The mutagenized plasmid DNA was transformed into a ΔlamB strain expressing OmpF315, a variant OmpF protein that confers a temperature-sensitive Dex+ phenotype (24). Transformed cells were spread on maltodextrin minimal medium containing BCIP to detect the alkaline phosphatase activity of PhoA (blue color), 0.2% l-arabinose for MzrA::PhoA expression, and ampicillin to select for plasmids. Expression of wild-type MzrA inhibits OmpF315 synthesis and causes a Dex− or down phenotype. The resulting small colonies appear blue due to normal PhoA synthesis from the plasmid. A null mutation in mzrA produces a polar effect on phoA, resulting in Dex+ PhoA− (white) colonies. The desired class of plasmid will carries a missense, nonpolar mutation in mzrA that interferes with the normal function of MzrA. These plasmids will result in Dex+ PhoA+ (blue) colonies. (B and C) Western blot analysis to determine OmpF and MzrA::PhoA levels. Proteins were analyzed from a strain expressing no MzrA (pBAD24), wild-type MzrA::PhoA, or its derivative containing single-amino-acid substitutions, as shown. OmpA (B) and AcrA (C) served as gel loading controls. In panel B, OmpF levels were quantified relative to those of OmpA, and the values were normalized to that determined from the pBAD24 vector control lane.